正在加载图片...
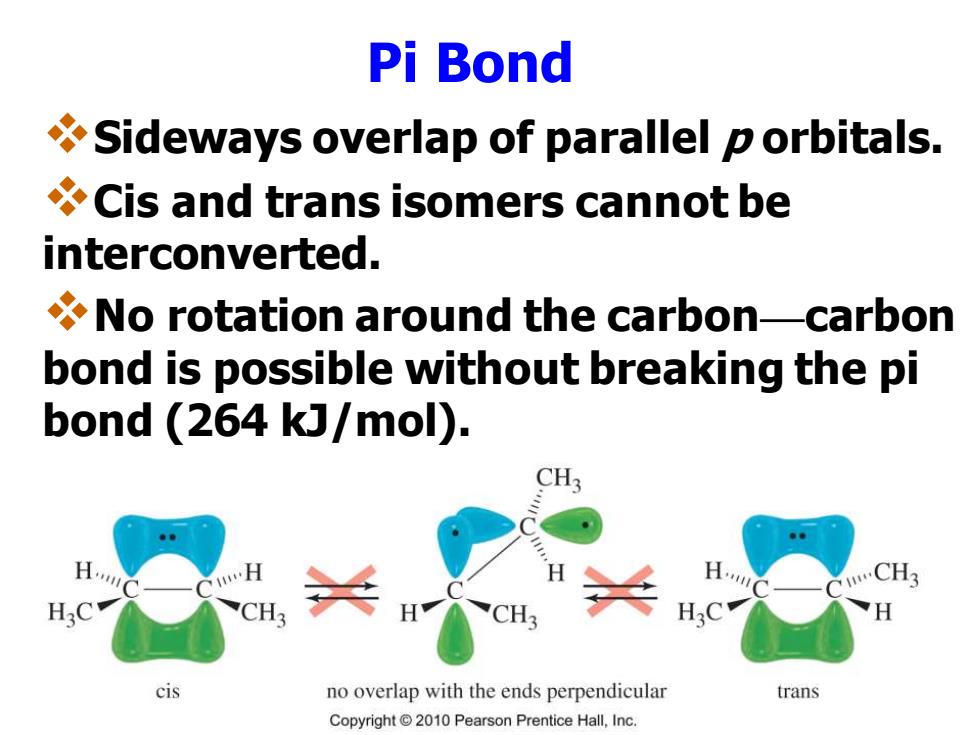
Pi Bond Sideways overlap of parallel p orbitals. Cis and trans isomers cannot be interconverted. No rotation around the carbon-carbon bond is possible without breaking the pi bond (264 kJ/mol). CH3 CH3 H:C CH cis no overlap with the ends perpendicular trans Copyright2010 Pearson Prentice Hall,Inc.Pi Bond ❖Sideways overlap of parallel p orbitals. ❖Cis and trans isomers cannot be interconverted. ❖No rotation around the carbon—carbon bond is possible without breaking the pi bond (264 kJ/mol)