正在加载图片...
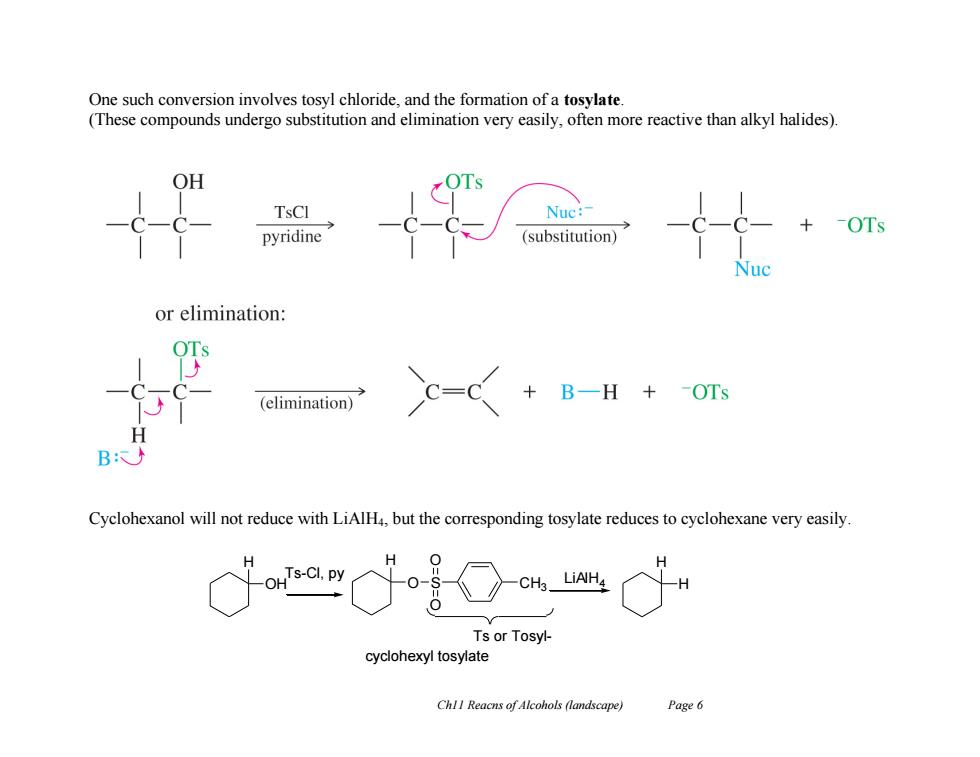
One such conversion involves tosyl chloride,and the formation of a tosylate (These compounds undergo substitution and elimination very easily,often more reactive than alkyl halides). OH TsCl Nuc: ·-OTs pyridine (substitution) or elimination: B一H+OTs (elimination) B: Cyclohexanol will not reduce with LiAlH,but the corresponding tosylate reduces to cyclohexane very easily. H OH Ts-CI,py 0- CHs LiAH Ts or Tosyl- cyclohexyl tosylate ChlI Reacns of Alcohols (landscape) Page 6 Ch11 Reacns of Alcohols (landscape) Page 6 One such conversion involves tosyl chloride, and the formation of a tosylate. (These compounds undergo substitution and elimination very easily, often more reactive than alkyl halides). Cyclohexanol will not reduce with LiAlH4, but the corresponding tosylate reduces to cyclohexane very easily. H OH H O S O O CH3 Ts or TosylTs-Cl, py cyclohexyl tosylate H H LiAlH4