正在加载图片...
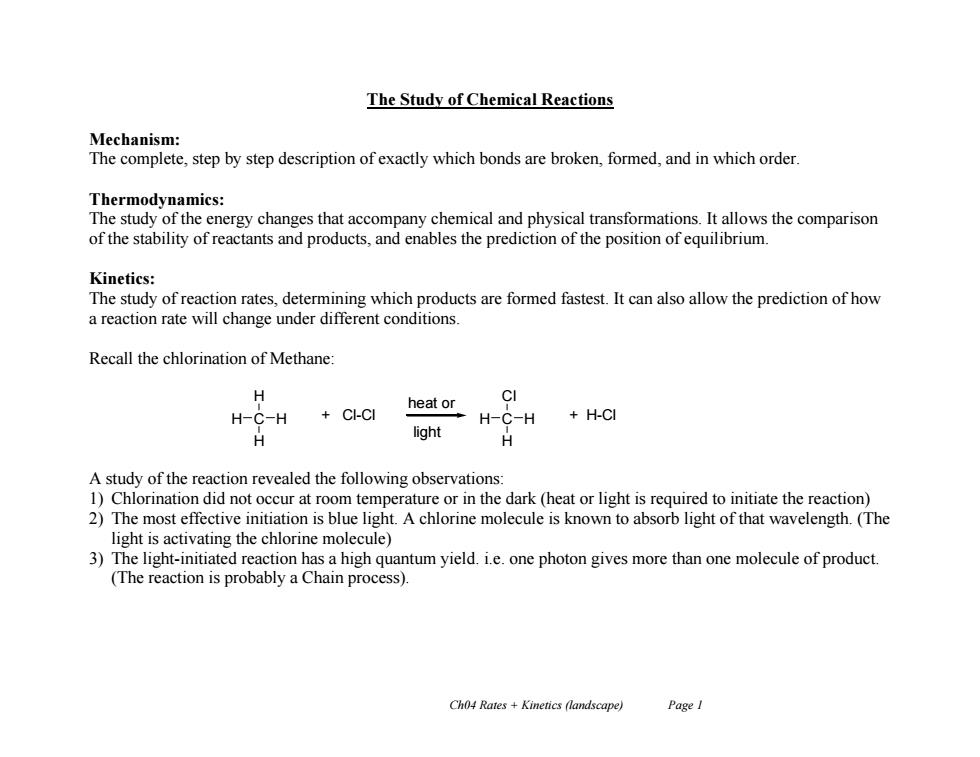
The Study of Chemical Reactions Mechanism: The complete,step by step description of exactly which bonds are broken,formed,and in which order. Thermodynamics: The study of the energy changes that accompany chemical and physical transformations.It allows the comparison of the stability of reactants and products,and enables the prediction of the position of equilibrium. Kinetics: The study of reaction rates,determining which products are formed fastest.It can also allow the prediction of how a reaction rate will change under different conditions. Recall the chlorination of Methane: CI heat or H-C-H CI-CI H-C-H +H-CI light H A study of the reaction revealed the following observations: 1)Chlorination did not occur at room temperature or in the dark (heat or light is required to initiate the reaction) 2)The most effective initiation is blue light.A chlorine molecule is known to absorb light of that wavelength.(The light is activating the chlorine molecule) 3)The light-initiated reaction has a high quantum yield.i.e.one photon gives more than one molecule of product. (The reaction is probably a Chain process). Ch04 Rates +Kinetics (landscape) PageICh04 Rates + Kinetics (landscape) Page 1 The Study of Chemical Reactions Mechanism: The complete, step by step description of exactly which bonds are broken, formed, and in which order. Thermodynamics: The study of the energy changes that accompany chemical and physical transformations. It allows the comparison of the stability of reactants and products, and enables the prediction of the position of equilibrium. Kinetics: The study of reaction rates, determining which products are formed fastest. It can also allow the prediction of how a reaction rate will change under different conditions. Recall the chlorination of Methane: A study of the reaction revealed the following observations: 1) Chlorination did not occur at room temperature or in the dark (heat or light is required to initiate the reaction) 2) The most effective initiation is blue light. A chlorine molecule is known to absorb light of that wavelength. (The light is activating the chlorine molecule) 3) The light-initiated reaction has a high quantum yield. i.e. one photon gives more than one molecule of product. (The reaction is probably a Chain process). H H C H H + Cl-Cl Cl H C H H + H-Cl heat or light