正在加载图片...
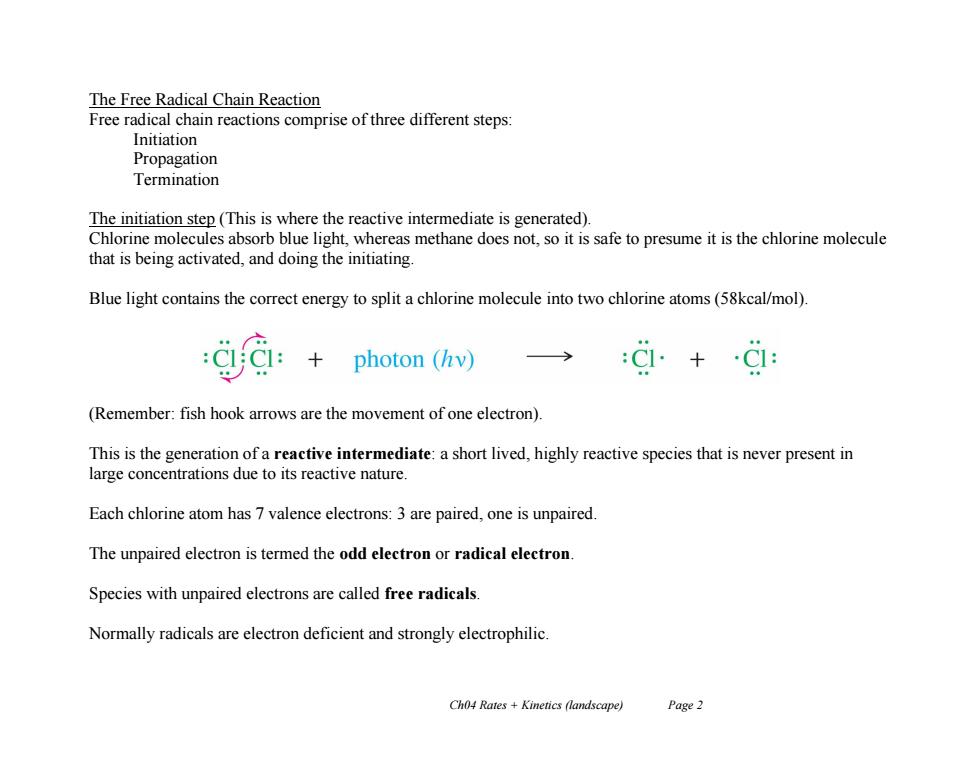
The Free Radical Chain Reaction Free radical chain reactions comprise of three different steps: Initiation Propagation Termination The initiation step (This is where the reactive intermediate is generated). Chlorine molecules absorb blue light,whereas methane does not,so it is safe to presume it is the chlorine molecule that is being activated,and doing the initiating. Blue light contains the correct energy to split a chlorine molecule into two chlorine atoms(58kcal/mol). photon (hv) → (Remember:fish hook arrows are the movement of one electron) This is the generation of a reactive intermediate:a short lived,highly reactive species that is never present in large concentrations due to its reactive nature. Each chlorine atom has 7 valence electrons:3 are paired,one is unpaired. The unpaired electron is termed the odd electron or radical electron Species with unpaired electrons are called free radicals. Normally radicals are electron deficient and strongly electrophilic. Ch04 Rates Kinetics (landscape) Page 2 Ch04 Rates + Kinetics (landscape) Page 2 The Free Radical Chain Reaction Free radical chain reactions comprise of three different steps: Initiation Propagation Termination The initiation step (This is where the reactive intermediate is generated). Chlorine molecules absorb blue light, whereas methane does not, so it is safe to presume it is the chlorine molecule that is being activated, and doing the initiating. Blue light contains the correct energy to split a chlorine molecule into two chlorine atoms (58kcal/mol). (Remember: fish hook arrows are the movement of one electron). This is the generation of a reactive intermediate: a short lived, highly reactive species that is never present in large concentrations due to its reactive nature. Each chlorine atom has 7 valence electrons: 3 are paired, one is unpaired. The unpaired electron is termed the odd electron or radical electron. Species with unpaired electrons are called free radicals. Normally radicals are electron deficient and strongly electrophilic