正在加载图片...
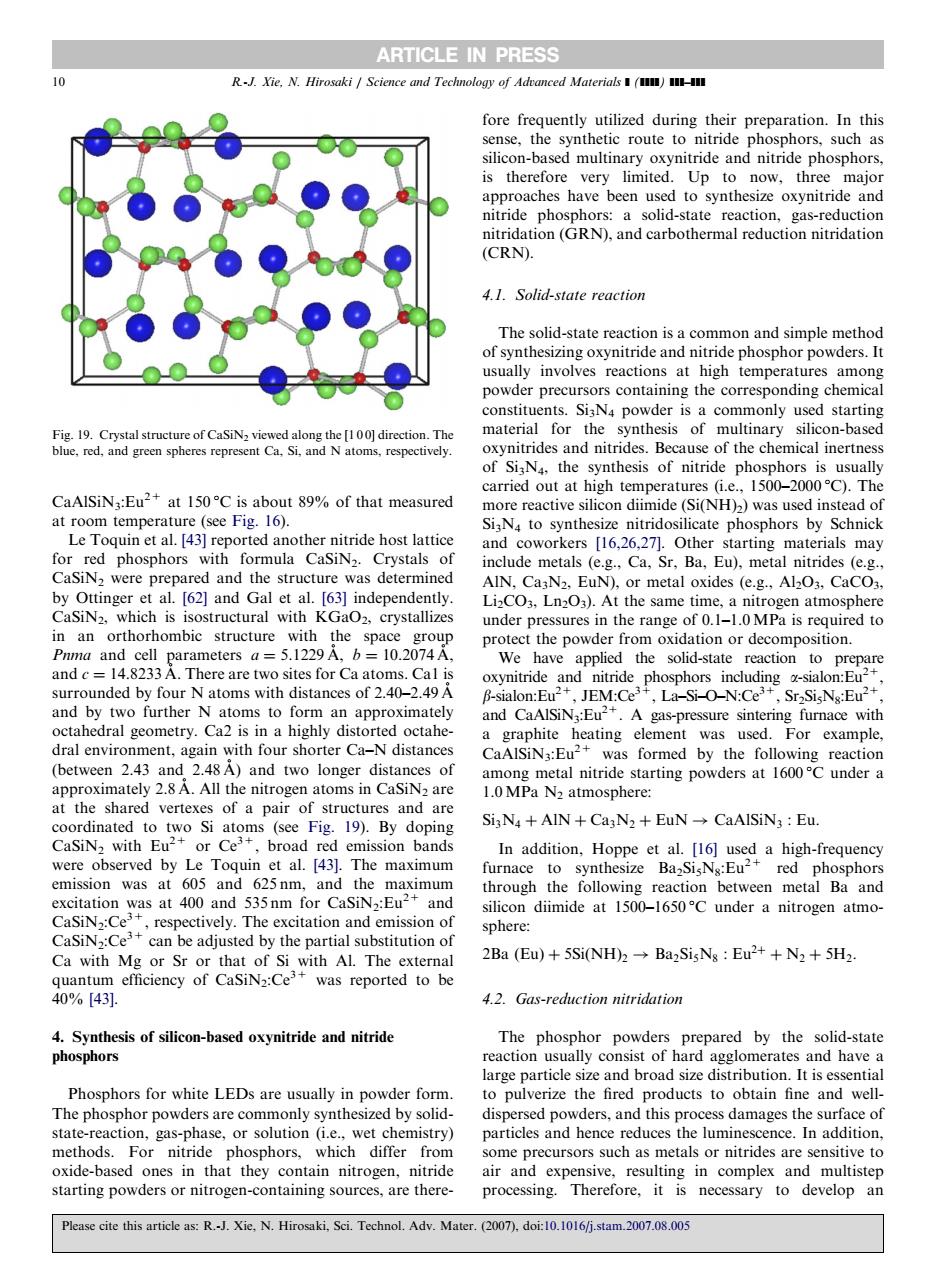
ARTICLE IN PRESS fore frequently utilized during their preparation.In this c route to nitride pho osphors.such as is therefor 品8p approaches have been used to synthesize oxynitride and t ).a (CRN) 4.1.Solid-state reaction The solid-staescommon simple method hor pow constituents.SisN4 powder is a commonly used starting ynth sis of mu tinary cal on-base of SiN the synthesis of nitride phost phors is usually 1500-2000C).Th CaAlSiN:Eu2+at 150C is about 89%of that measured carried out at high temperatures(i.e Si(NH)2)was used 1d0 at r Le Toquin et al.[43]reported another nitride host lattice byials may for red phosphors with include metals (e.g..Ca.Sr.Ba.Eu).metal nitrides (e.g. 162 and Gal AIN. CasN2.EuN),or metal oxides (e.g.,Al2O3,CaCO CaSiNz which is isostructural with KGaoz.crystallizes 3.Ln2O3). the samet 0 1 10MR in an orthorhombic structure with the space fepoderionoidationor'decomposSton We have applied the solid-state reaction to prepre surrounded by four N atoms with distances of 240-2.49A oxynitr d pho and by two further N atoms to form an approximately nressure sintering furnace with edral geometry. dral e Ca- sen 2 43 and 248A)and two long under a approximately 2.8A.All the nitrogen atoms in CaSiNs are at the sharec are SigNa AIN +CagN2 EuN CaAlSiN3 Eu. were observed by Le Toquin et al.[43].The maximum emission was 625nm through the following reaction between metal Ba and 40 anc silicon diimide at 1500-1650C under a nitrogen atmo- sphere Ca with Mg or Sr or that of Si with Al.The external 2Ba(Eu+5SiNH2→Ba2 SisNs:Eu2++N2+5H2 was reported to be 4.2.Gas-reduction nitridation 4.Synthesis of silicon-based oxynitride and nitride The phosphor powders prepared by the solid-state phosphors Phosphors for white lEDs epartic dis and this n ss damages the surface of particles and hence reduces the luminescence.In addition. methods. For nitride phosphors, whic some precursors such as metals or nitrides are sensitive to rogen- ing sources,a an Please cite this articles:RJ.Xie,N.Hirosaki,Sci.Technol.Adv.Mater.(do:.0 CaAlSiN3:Eu2+ at 150 1C is about 89% of that measured at room temperature (see Fig. 16). Le Toquin et al. [43] reported another nitride host lattice for red phosphors with formula CaSiN2. Crystals of CaSiN2 were prepared and the structure was determined by Ottinger et al. [62] and Gal et al. [63] independently. CaSiN2, which is isostructural with KGaO2, crystallizes in an orthorhombic structure with the space group Pnma and cell parameters a ¼ 5.1229 A˚ , b ¼ 10.2074 A˚ , and c ¼ 14.8233 A˚ . There are two sites for Ca atoms. Ca1 is surrounded by four N atoms with distances of 2.40–2.49 A˚ and by two further N atoms to form an approximately octahedral geometry. Ca2 is in a highly distorted octahedral environment, again with four shorter Ca–N distances (between 2.43 and 2.48 A˚ ) and two longer distances of approximately 2.8 A˚ . All the nitrogen atoms in CaSiN2 are at the shared vertexes of a pair of structures and are coordinated to two Si atoms (see Fig. 19). By doping CaSiN2 with Eu2+ or Ce3+, broad red emission bands were observed by Le Toquin et al. [43]. The maximum emission was at 605 and 625 nm, and the maximum excitation was at 400 and 535 nm for CaSiN2:Eu2+ and CaSiN2:Ce3+, respectively. The excitation and emission of CaSiN2:Ce3+ can be adjusted by the partial substitution of Ca with Mg or Sr or that of Si with Al. The external quantum efficiency of CaSiN2:Ce3+ was reported to be 40% [43]. 4. Synthesis of silicon-based oxynitride and nitride phosphors Phosphors for white LEDs are usually in powder form. The phosphor powders are commonly synthesized by solidstate-reaction, gas-phase, or solution (i.e., wet chemistry) methods. For nitride phosphors, which differ from oxide-based ones in that they contain nitrogen, nitride starting powders or nitrogen-containing sources, are therefore frequently utilized during their preparation. In this sense, the synthetic route to nitride phosphors, such as silicon-based multinary oxynitride and nitride phosphors, is therefore very limited. Up to now, three major approaches have been used to synthesize oxynitride and nitride phosphors: a solid-state reaction, gas-reduction nitridation (GRN), and carbothermal reduction nitridation (CRN). 4.1. Solid-state reaction The solid-state reaction is a common and simple method of synthesizing oxynitride and nitride phosphor powders. It usually involves reactions at high temperatures among powder precursors containing the corresponding chemical constituents. Si3N4 powder is a commonly used starting material for the synthesis of multinary silicon-based oxynitrides and nitrides. Because of the chemical inertness of Si3N4, the synthesis of nitride phosphors is usually carried out at high temperatures (i.e., 1500–2000 1C). The more reactive silicon diimide (Si(NH)2) was used instead of Si3N4 to synthesize nitridosilicate phosphors by Schnick and coworkers [16,26,27]. Other starting materials may include metals (e.g., Ca, Sr, Ba, Eu), metal nitrides (e.g., AlN, Ca3N2, EuN), or metal oxides (e.g., Al2O3, CaCO3, Li2CO3, Ln2O3). At the same time, a nitrogen atmosphere under pressures in the range of 0.1–1.0 MPa is required to protect the powder from oxidation or decomposition. We have applied the solid-state reaction to prepare oxynitride and nitride phosphors including a-sialon:Eu2+, b-sialon:Eu2+, JEM:Ce3+, La–Si–O–N:Ce3+, Sr2Si5N8:Eu2+, and CaAlSiN3:Eu2+. A gas-pressure sintering furnace with a graphite heating element was used. For example, CaAlSiN3:Eu2+ was formed by the following reaction among metal nitride starting powders at 1600 1C under a 1.0 MPa N2 atmosphere: Si3N4 þ AlN þ Ca3N2 þ EuN ! CaAlSiN3 : Eu: In addition, Hoppe et al. [16] used a high-frequency furnace to synthesize Ba2Si5N8:Eu2+ red phosphors through the following reaction between metal Ba and silicon diimide at 1500–1650 1C under a nitrogen atmosphere: 2Ba ðEuÞ þ 5SiðNHÞ2 ! Ba2Si5N8 : Eu2þ þ N2 þ 5H2: 4.2. Gas-reduction nitridation The phosphor powders prepared by the solid-state reaction usually consist of hard agglomerates and have a large particle size and broad size distribution. It is essential to pulverize the fired products to obtain fine and welldispersed powders, and this process damages the surface of particles and hence reduces the luminescence. In addition, some precursors such as metals or nitrides are sensitive to air and expensive, resulting in complex and multistep processing. Therefore, it is necessary to develop an ARTICLE IN PRESS Fig. 19. Crystal structure of CaSiN2 viewed along the [1 0 0] direction. The blue, red, and green spheres represent Ca, Si, and N atoms, respectively. 10 R.-J. Xie, N. Hirosaki / Science and Technology of Advanced Materials ] (]]]]) ]]]–]]] Please cite this article as: R.-J. Xie, N. Hirosaki, Sci. Technol. Adv. Mater. (2007), doi:10.1016/j.stam.2007.08.005