正在加载图片...
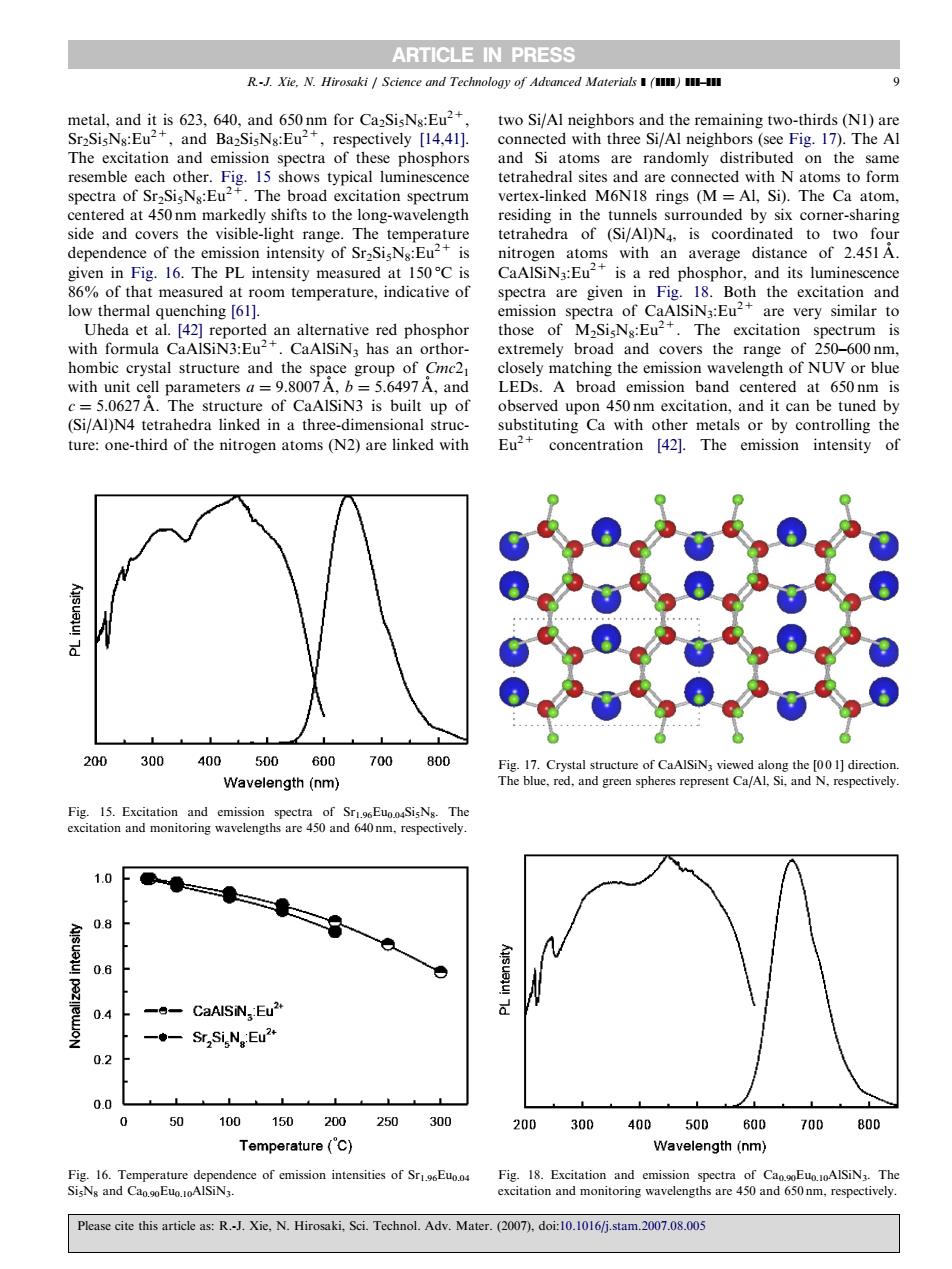
ARTICLE IN PRESS metal.and it is 623.640.and 650nm for Ca-SisNs:Eu2 two Si/Al neighbors and the remaining two-thirds (N1)are Sr2SisNs:Eu2+,and BazSisNg:Eu2 respectively [14.41]. connected with three Si/Al neighbors(see Fig.17).The Al of thes e phosphor and Si atoms are randomly distribu d on the same etr ex-linked M6NI8 rings (MAL Si neredmarkedlyshstth onwaveleng side and covers the visible-light range The temperature depe 16.The of an average d low thermal quenching [61]. are very similar to 1215N8 excitation with fo homhic crystal structure and the lparametersSiN s bul and LEDs.A broad emission band centered at 650nm is The str of CaAlSIN3 is built up o :on-third of the m a tre e linked wi 200300400 500600 700800 Wavelength (nm) blue.red.and green spheres represe 1.0 ● 0 -CaAISIN Eu" -Sr2Si,N,Eu2* 00 0 50 100150200250 300 200 300 50n 00 700 800 Temperature ('C) Wavelength(nm Please cite this article as:R.Xie,N.Hirosaki,Sci.Technol.Adv.Mater.(007).do:1016/-stam.2007.0.005metal, and it is 623, 640, and 650 nm for Ca2Si5N8:Eu2+, Sr2Si5N8:Eu2+, and Ba2Si5N8:Eu2+, respectively [14,41]. The excitation and emission spectra of these phosphors resemble each other. Fig. 15 shows typical luminescence spectra of Sr2Si5N8:Eu2+. The broad excitation spectrum centered at 450 nm markedly shifts to the long-wavelength side and covers the visible-light range. The temperature dependence of the emission intensity of Sr2Si5N8:Eu2+ is given in Fig. 16. The PL intensity measured at 150 1C is 86% of that measured at room temperature, indicative of low thermal quenching [61]. Uheda et al. [42] reported an alternative red phosphor with formula CaAlSiN3:Eu2+. CaAlSiN3 has an orthorhombic crystal structure and the space group of Cmc21 with unit cell parameters a ¼ 9.8007 A˚ , b ¼ 5.6497 A˚ , and c ¼ 5.0627 A˚ . The structure of CaAlSiN3 is built up of (Si/Al)N4 tetrahedra linked in a three-dimensional structure: one-third of the nitrogen atoms (N2) are linked with two Si/Al neighbors and the remaining two-thirds (N1) are connected with three Si/Al neighbors (see Fig. 17). The Al and Si atoms are randomly distributed on the same tetrahedral sites and are connected with N atoms to form vertex-linked M6N18 rings (M ¼ Al, Si). The Ca atom, residing in the tunnels surrounded by six corner-sharing tetrahedra of (Si/Al)N4, is coordinated to two four nitrogen atoms with an average distance of 2.451 A˚ . CaAlSiN3:Eu2+ is a red phosphor, and its luminescence spectra are given in Fig. 18. Both the excitation and emission spectra of CaAlSiN3:Eu2+ are very similar to those of M2Si5N8:Eu2+. The excitation spectrum is extremely broad and covers the range of 250–600 nm, closely matching the emission wavelength of NUV or blue LEDs. A broad emission band centered at 650 nm is observed upon 450 nm excitation, and it can be tuned by substituting Ca with other metals or by controlling the Eu2+ concentration [42]. The emission intensity of ARTICLE IN PRESS Fig. 16. Temperature dependence of emission intensities of Sr1.96Eu0.04 Si5N8 and Ca0.90Eu0.10AlSiN3. Fig. 18. Excitation and emission spectra of Ca0.90Eu0.10AlSiN3. The excitation and monitoring wavelengths are 450 and 650 nm, respectively. Fig. 15. Excitation and emission spectra of Sr1.96Eu0.04Si5N8. The excitation and monitoring wavelengths are 450 and 640 nm, respectively. Fig. 17. Crystal structure of CaAlSiN3 viewed along the [0 0 1] direction. The blue, red, and green spheres represent Ca/Al, Si, and N, respectively. R.-J. Xie, N. Hirosaki / Science and Technology of Advanced Materials ] (]]]]) ]]]–]]] 9 Please cite this article as: R.-J. Xie, N. Hirosaki, Sci. Technol. Adv. Mater. (2007), doi:10.1016/j.stam.2007.08.005