正在加载图片...
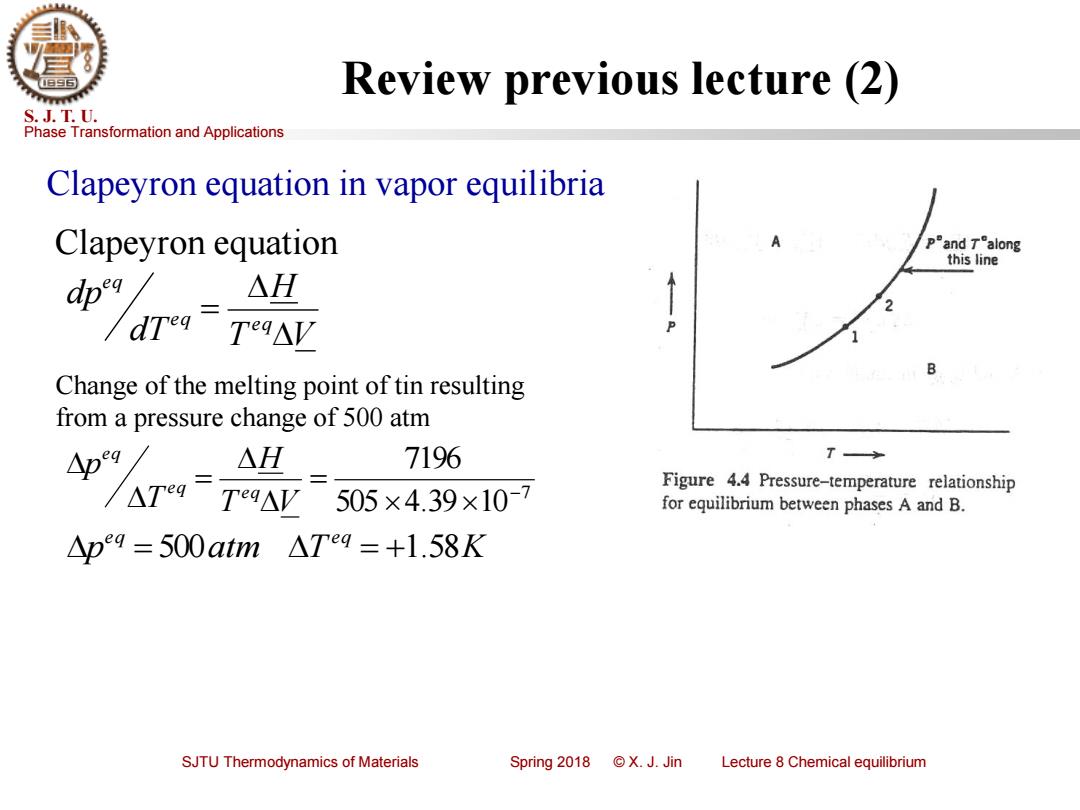
Review previous lecture (2) S.J.T.U. Phase Transformation and Applications Clapeyron equation in vapor equilibria Clapeyron equation pand Talong this line △H Teg△V B Change of the melting point of tin resulting from a pressure change of 500 atm 人 AH 7196 T Figure 4.4 Pressure-temperature relationship Teg△V 505×4.39×10-7 for equilibrium between phases A and B. △p9=500atm△Teg=+1.58K SJTU Thermodynamics of Materials Spring2018©X.J.Jin Lecture 8 Chemical equilibriumPhase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Spring 2018 © X. J. Jin Lecture 8 Chemical equilibrium Review previous lecture (2) Clapeyron equation in vapor equilibria T V H dT dp e q e q e q = Clapeyron equation Change of the melting point of tin resulting from a pressure change of 500 atm 7 505 4.39 10 7196 − = = T V H T p e q e q e q p atm T K e q e q = 500 = +1.58