正在加载图片...
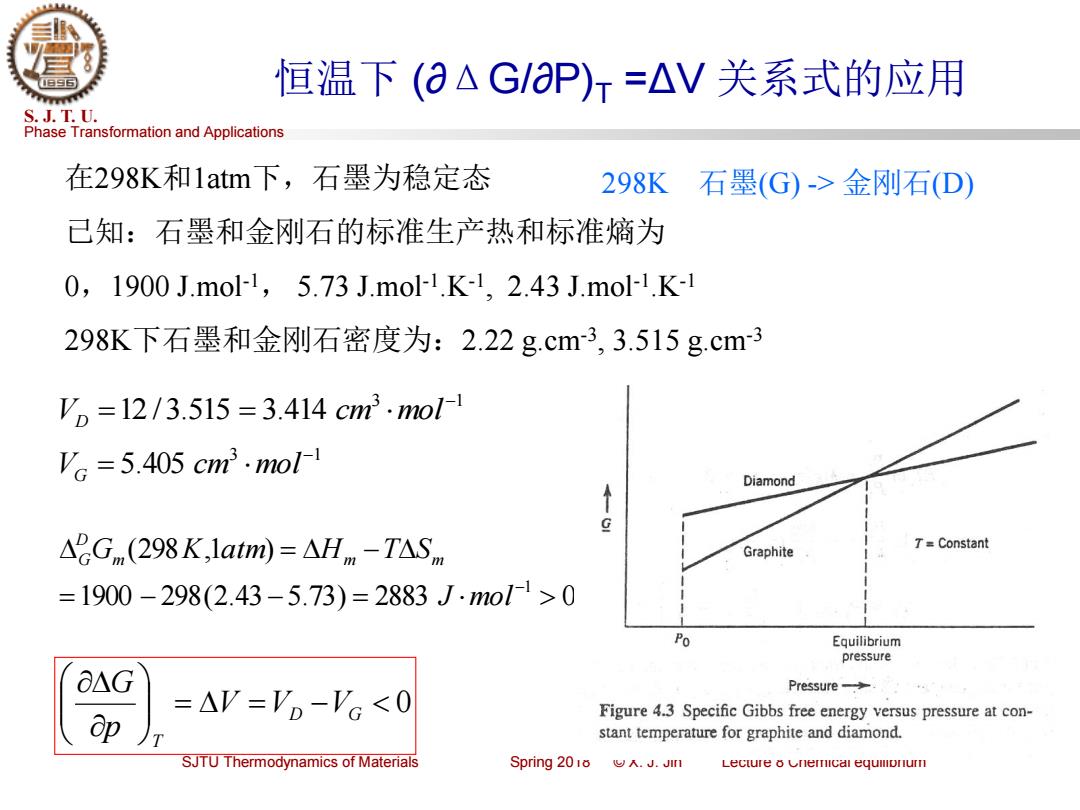
恒温下(aAG/aP)r=△V关系式的应用 S.J.T.U. Phase Transformation and Applications 在298K和1atm下,石墨为稳定态 298K 石墨(G)->金刚石(D) 已知:石墨和金刚石的标准生产热和标准熵为 0,1900J.mol1,5.73J.mol1.K-1,2.43J.mo1.K-1 298K下石墨和金刚石密度为:2.22g.cm-3,3.515g.cm3 V,=12/3.515=3.414cm3.mol1 'c=5.405cm3.mol1 Diamond ↑ g △2Gnm(298K,1atm)=△Hm-T△S,m Graphite 7=Constant =1900-298(2.43-5.73)=2883J.mo1>0 Po Equilibrium pressure O△G Pressure-> =AV='o-'c<0 op Figure 4.3 Specific Gibbs free energy versus pressure at con- stant temperature for graphite and diamond. SJTU Thermodynamics of Materials Spring 2018.J.Jin Lecture 8 Cnemical equllonurPhase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Spring 2018 © X. J. Jin Lecture 8 Chemical equilibrium 恒温下 (∂ΔG/∂P)T =ΔV 关系式的应用 在298K和1atm下,石墨为稳定态 已知:石墨和金刚石的标准生产热和标准熵为 0,1900 J.mol-1 , 5.73 J.mol-1 .K-1 , 2.43 J.mol-1 .K-1 298K下石墨和金刚石密度为:2.22 g.cm-3 , 3.515 g.cm-3 = = − 0 D G T V V V p G 298K 石墨(G) -> 金刚石(D) 1900 298(2.43 5.73) 2883 0 (298 ,1 ) 1 = − − = = − − J mol Gm K atm Hm T Sm D G 3 1 12 /3.515 3.414 − V = = cm mol D 3 1 5.405 − V = cm mol G