正在加载图片...
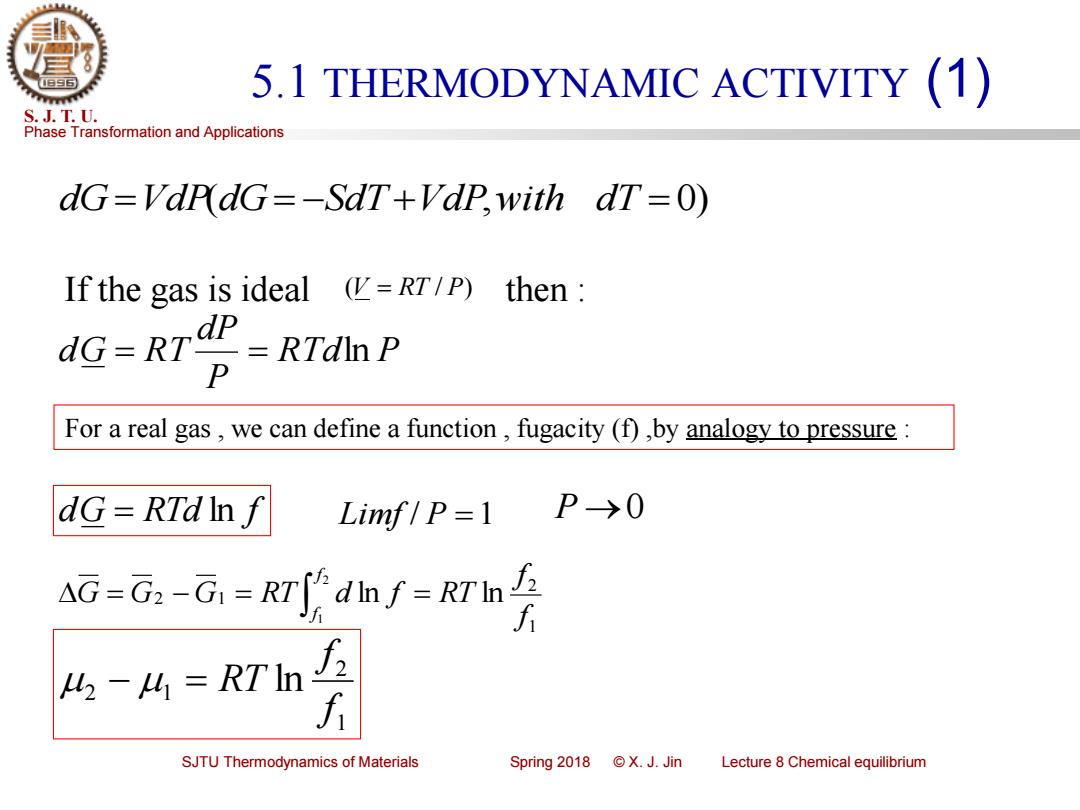
5.1 THERMODYNAMIC ACTIVITY (1) S.J.T.U. Phase Transformation and Applications dG=VdrdG=-SdT+Vdp,with dT=0) If the gas is ideal (RT/P) then dp dG=RT=RTdln P For a real gas,we can define a function,fugacity (f),by analogy to pressure: dG=RTdInf Limf/P=1 P→0 AG=G:-G-RTJdIn /-RTIn f t-M=RTIn f SJTU Thermodynamics of Materials Spring2018©X.J.Jin Lecture 8 Chemical equilibriumPhase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Spring 2018 © X. J. Jin Lecture 8 Chemical equilibrium 5.1 THERMODYNAMIC ACTIVITY (1) dG=VdP(dG= −SdT +VdP,with dT = 0) If the gas is ideal then : (V = RT / P) RTd P P dP dG = RT = ln For a real gas , we can define a function , fugacity (f) ,by analogy to pressure : dG = RTd ln f Limf / P =1 P →0 1 2 2 1 ln ln 2 1 f f G G G RT d f RT f f = − = = 1 2 2 1 ln f f − = RT