正在加载图片...
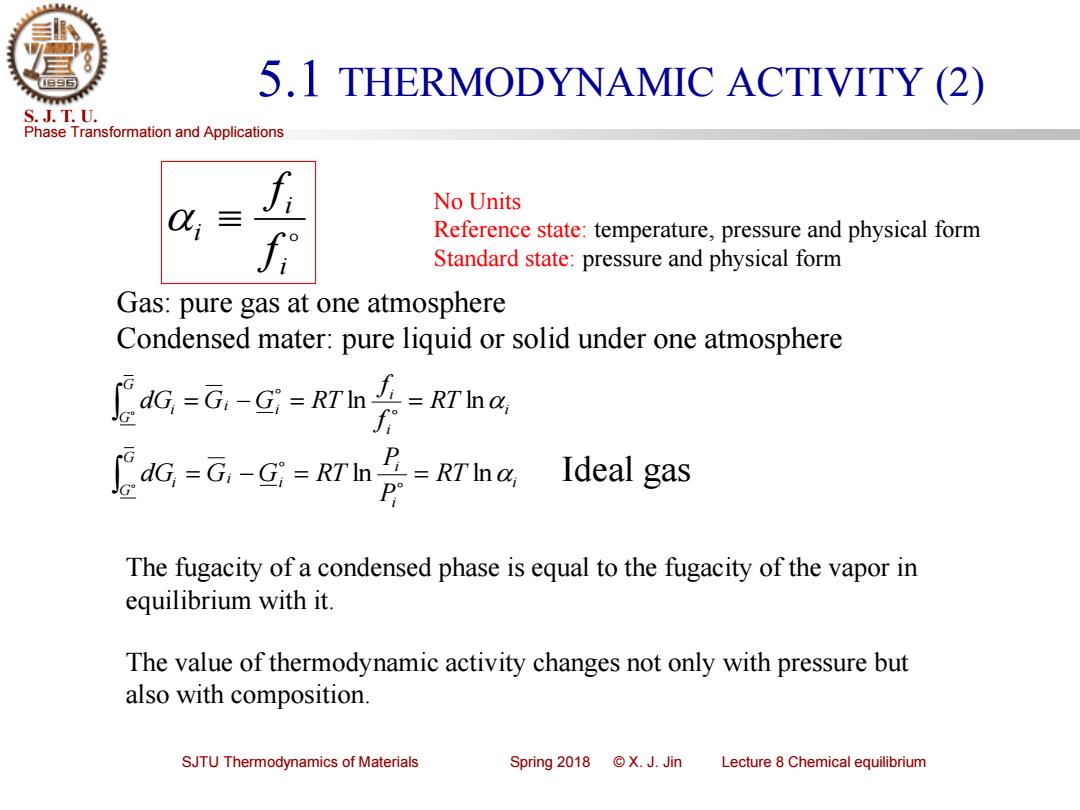
5.1 THERMODYNAMIC ACTIVITY (2) S.J.T.U. Phase Transformation and Applications No Units 三 Ji Reference state:temperature,pressure and physical form Standard state:pressure and physical form Gas:pure gas at one atmosphere Condensed mater:pure liquid or solid under one atmosphere dG,=G,-Gj-KTIn-RT'Ina dG,-G,-G=RTIn P=RTIna Ideal gas The fugacity of a condensed phase is equal to the fugacity of the vapor in equilibrium with it. The value of thermodynamic activity changes not only with pressure but also with composition. SJTU Thermodynamics of Materials Spring2018©X.J.Jin Lecture 8 Chemical equilibriumPhase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Spring 2018 © X. J. Jin Lecture 8 Chemical equilibrium 5.1 THERMODYNAMIC ACTIVITY (2) i i i f f i i i i i G G i RT f f dG = G − G = RT ln = ln i i i i i G G i RT P P dG = G − G = RT ln = ln Ideal gas No Units Reference state: temperature, pressure and physical form Standard state: pressure and physical form Gas: pure gas at one atmosphere Condensed mater: pure liquid or solid under one atmosphere The fugacity of a condensed phase is equal to the fugacity of the vapor in equilibrium with it. The value of thermodynamic activity changes not only with pressure but also with composition