正在加载图片...
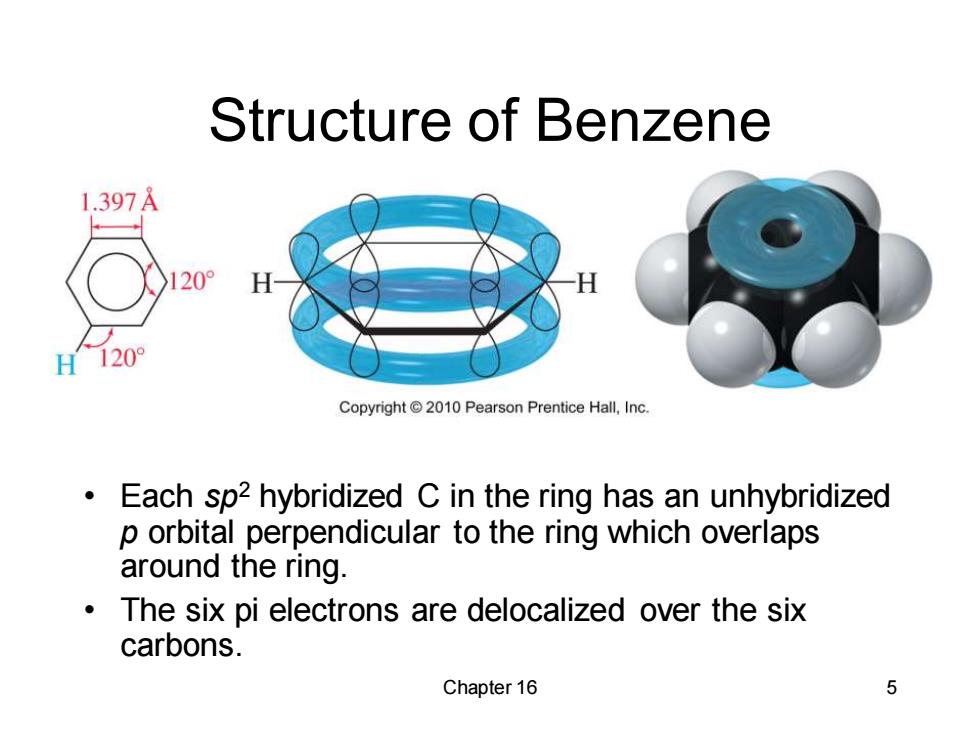
Structure of Benzene 1.397A 20° H 120° Copyright 2010 Pearson Prentice Hall,Inc. Each sp2 hybridized C in the ring has an unhybridized p orbital perpendicular to the ring which overlaps around the ring. The six pi electrons are delocalized over the six carbons. Chapter 16 5Chapter 16 5 Structure of Benzene • Each sp2 hybridized C in the ring has an unhybridized p orbital perpendicular to the ring which overlaps around the ring. • The six pi electrons are delocalized over the six carbons