正在加载图片...
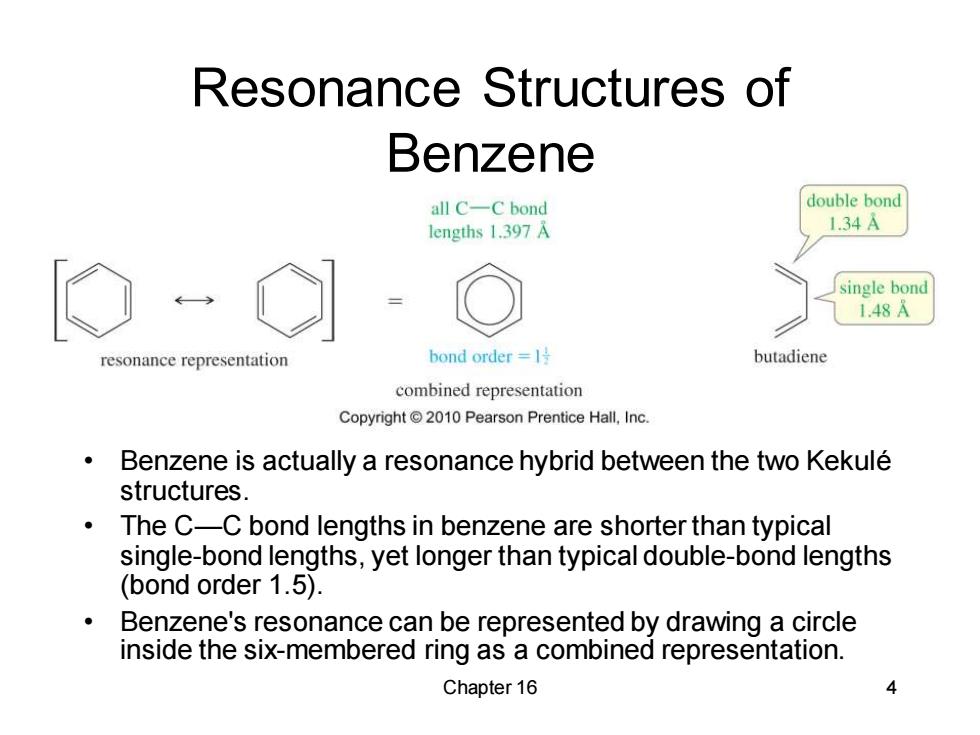
Resonance Structures of Benzene all C-C bond double bond lengths 1.397 A 1.34A single bond 1.48A resonance representation bond order=l号 butadiene combined representation Copyright 2010 Pearson Prentice Hall,Inc. Benzene is actually a resonance hybrid between the two Kekule structures. The C-C bond lengths in benzene are shorter than typical single-bond lengths,yet longer than typical double-bond lengths (bond order 1.5). Benzene's resonance can be represented by drawing a circle inside the six-membered ring as a combined representation. Chapter 16 Chapter 16 4 Resonance Structures of Benzene • Benzene is actually a resonance hybrid between the two Kekulé structures. • The C—C bond lengths in benzene are shorter than typical single-bond lengths, yet longer than typical double-bond lengths (bond order 1.5). • Benzene's resonance can be represented by drawing a circle inside the six-membered ring as a combined representation