正在加载图片...
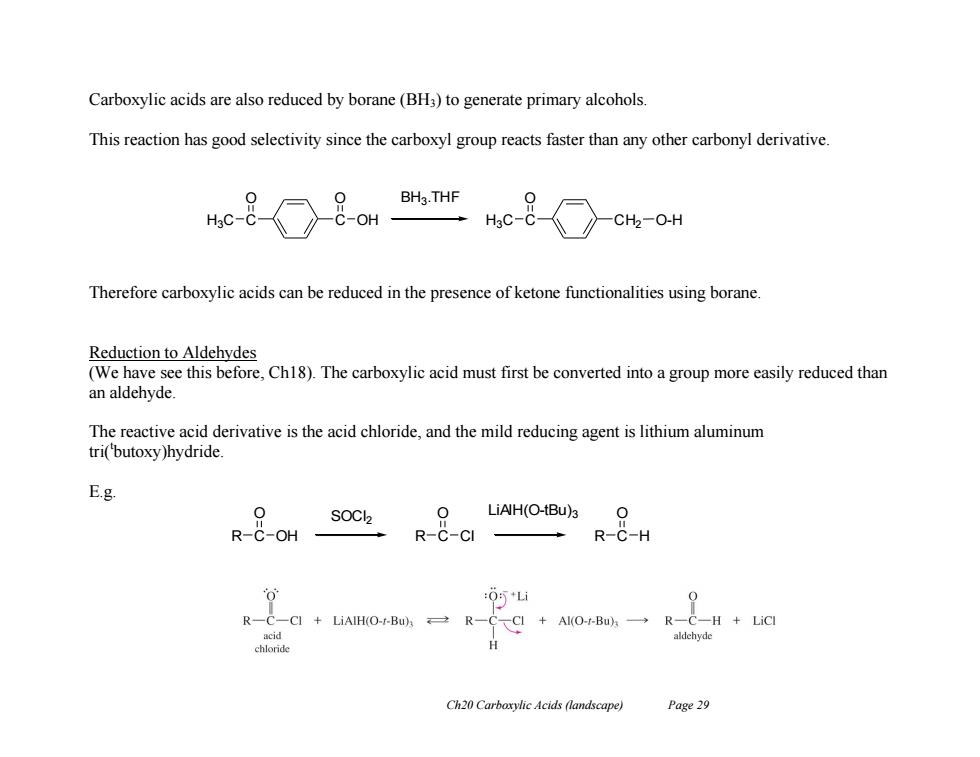
Carboxylic acids are also reduced by borane(BH3)to generate primary alcohols. This reaction has good selectivity since the carboxyl group reacts faster than any other carbonyl derivative. BH3.THF -OH H3C-( CH2-O-H Therefore carboxylic acids can be reduced in the presence of ketone functionalities using borane. Reduction to Aldehydes (We have see this before,Ch18).The carboxylic acid must first be converted into a group more easily reduced than an aldehyde. The reactive acid derivative is the acid chloride,and the mild reducing agent is lithium aluminum tri(butoxy)hydride. E.g. 0 SOCl2 iANH(O-Bu为O 0 R-C-OH R-C-CI R-C-H :0*Li 0 R-C-CI+LiAIH(O-rBuA→之R-C、CI+AI(O-tBuA一→R一C-H+LiCI acid aldehyde chloride H Ch20 Carboxylic Acids (landscape) Page 29Ch20 Carboxylic Acids (landscape) Page 29 Carboxylic acids are also reduced by borane (BH3) to generate primary alcohols. This reaction has good selectivity since the carboxyl group reacts faster than any other carbonyl derivative. Therefore carboxylic acids can be reduced in the presence of ketone functionalities using borane. Reduction to Aldehydes (We have see this before, Ch18). The carboxylic acid must first be converted into a group more easily reduced than an aldehyde. The reactive acid derivative is the acid chloride, and the mild reducing agent is lithium aluminum tri(t butoxy)hydride. E.g. C C O H3C O OH C CH2 O H3C O-H BH3 .THF R C O OH SOCl2 R C O Cl LiAlH(O-tBu)3 R C O H