正在加载图片...
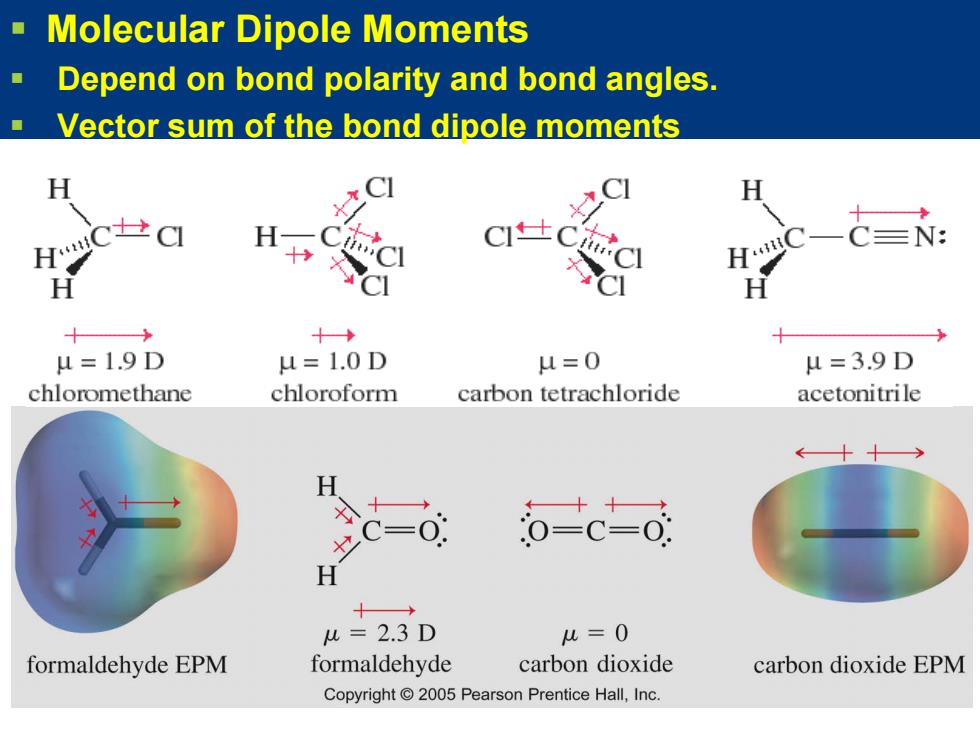
Molecular Dipole Moments Depend on bond polarity and bond angles. Vector sum of the bond dipole moments H CI CI H C=N: 十 H C】 CI 十 十→ u=1.9D u=1.0D u=0 u=3.9D chloromethane chloroform carbon tetrachloride acetonitrile H =0 o-c-o: H 十→ u=2.3D u=0 formaldehyde EPM formaldehyde carbon dioxide carbon dioxide EPM Copyright 2005 Pearson Prentice Hall,Inc. Molecular Dipole Moments Depend on bond polarity and bond angles. Vector sum of the bond dipole moments