正在加载图片...
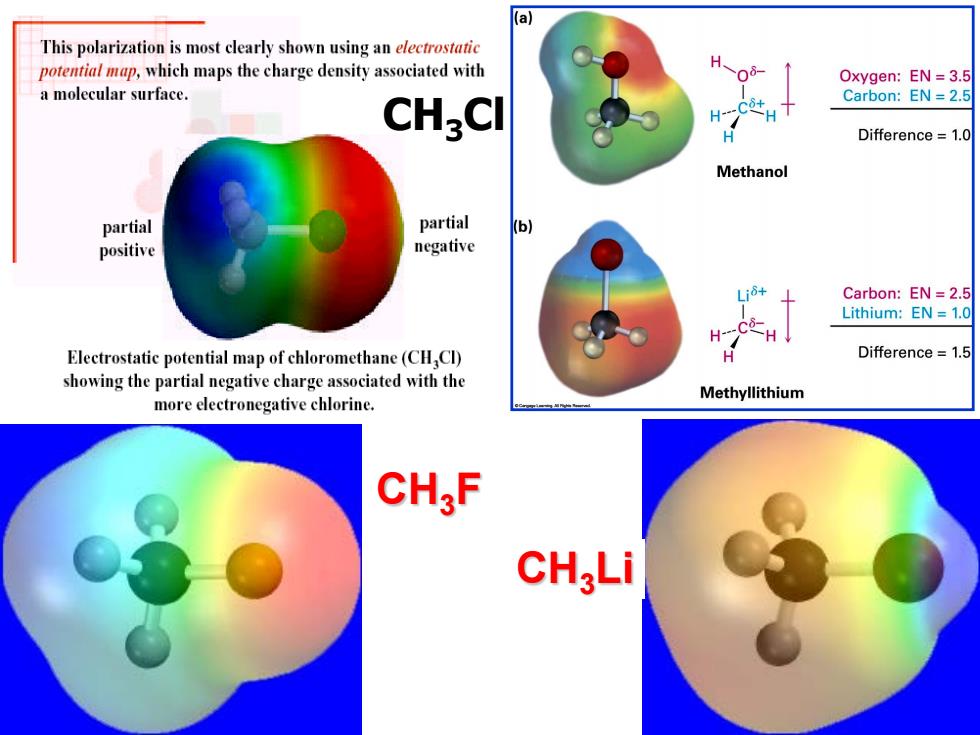
(a This polarization is most clearly shown using an electrostatic potential map,which maps the charge density associated with H Oxygen:EN=3.5 a molecular surface. Carbon:EN=2.5 CHgCl H---C+ Difference=1.0 Methanol partial partial (b) positive negative Carbon:EN =2.5 Lithium:EN 1.0 H Electrostatic potential map of chloromethane(CH Cl) Difference =1.5 showing the partial negative charge associated with the Methyllithium more electronegative chlorine. CH,F CH;LiCH3Cl CH3F CH3Li