正在加载图片...
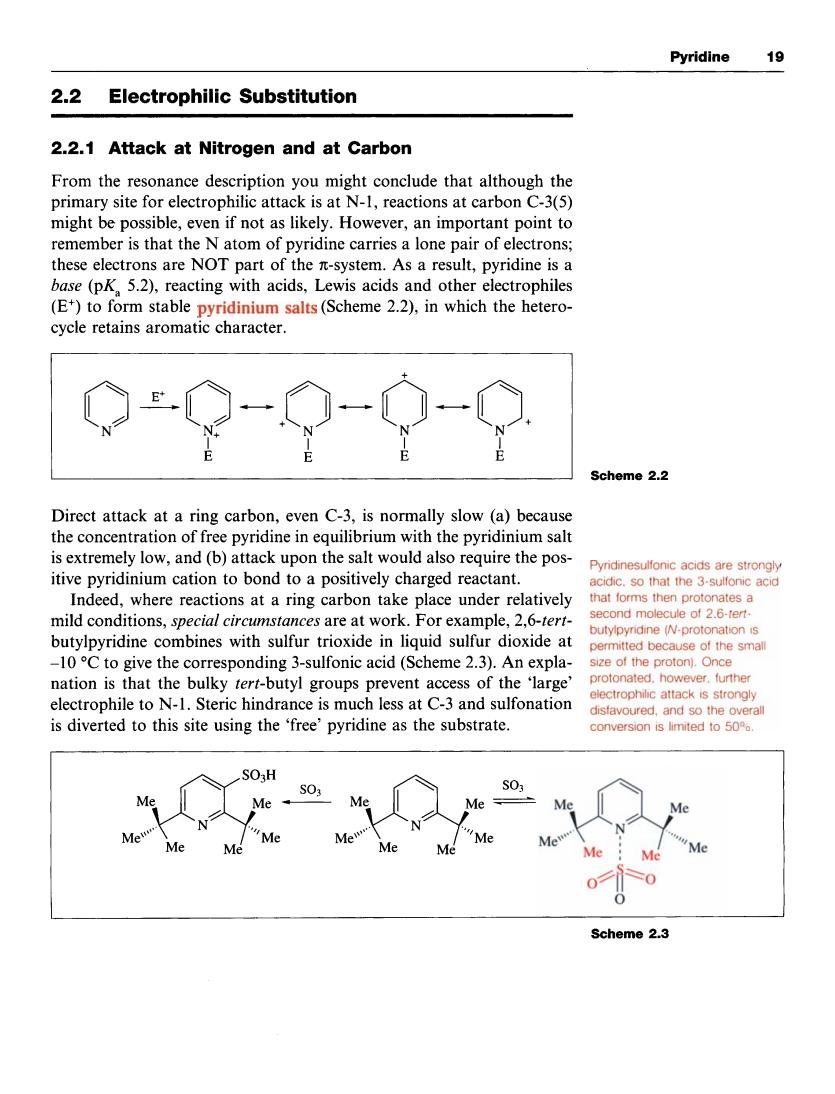
Pyridine 19 2.2 Electrophilic Substitution 2.2.1 Attack at Nitrogen and at Carbon From the resonance description you might conclude that although the primary site for electrophilic attack is at N-1,reactions at carbon C-3(5) might be possible,even if not as likely.However,an important point to remember is that the N atom of pyridine carries a lone pair of electrons; these electrons are NOT part of the n-system.As a result,pyridine is a base(pK.5.2).reacting with acids,Lewis acids and other electrophiles me2.2 Direct attack at a ring carbon,even C-3,is normally slow (a)because the concentration of free pyridine in equilibrium with the pyridinium salt is extremely low,and(b)attack upon the salt would also require the pos- Pyndinesulfonic acids are strongly itive pyridinium cation to bond to a positively charged reactant Indeed,where reactions at a ring carbon take place under relatively mild conditions,special circumstances are at work.For example,2,6-tert- butylpyndine N-protonaton is butylpyridine combines with sulfur trioxide in liquid sulfur dioxide at -10C to give the corresponding 3-sulfonic acid(Scheme 2.3).An expla nation is that the bulky tert-butyl groups prevent access of the 'large' electrophile to N-1.Steric hindrance is much less at C-3 and sulfonation ectrophlc attacks strongly is diverted to this site using the 'free'pyridine as the substrate. SO3H S03 Me Me M Me Me Scheme 2.3Pyridine I9 2.2 Electrophilic Substitution 2.2.1 Attack at Nitrogen and at Carbon From the resonance description you might conclude that although the primary site for electrophilic attack is at N-1, reactions at carbon C-3(5) might be possible, even if not as likely. However, an important point to remember is that the N atom of pyridine carries a lone pair of electrons; these electrons are NOT part of the n;-system. As a result, pyridine is a base (pKa 5.2), reacting with acids, Lewis acids and other electrophiles (E') to form stable pyridinium salts (Scheme 2.2), in which the heterocycle retains aromatic character. 0 0 -,o N - 0 N - o+ N Y+ I I I N E E E E Direct attack at a ring carbon, even C-3, is normally slow (a) because the concentration of free pyridine in equilibrium with the pyridinium salt is extremely low, and (b) attack upon the salt would also require the positive pyridinium cation to bond to a positively charged reactant. Indeed, where reactions at a ring carbon take place under relatively mild conditions, special circumstances are at work. For example, 2,6-tertbutylpyridine combines with sulfur trioxide in liquid sulfur dioxide at -10 "C to give the corresponding 3-sulfonic acid (Scheme 2.3). An explanation is that the bulky tert-butyl groups prevent access of the 'large' electrophile to N- 1. Steric hindrance is much less at C-3 and sulfonation is diverted to this site using the 'free' pyridine as the substrate. Scheme 2.2 Pyridinesulfonic acids are strongly acidic, so that the 3-sulfonic acid that forms then protonates a second molecule of 2,6-tertbutylpyridine (N-protonation is permitted because of the small size of the proton). Once protonated, however, further electrophilic attack is strongly disfavoured, and so the overall conversion is limited to 50%. Scheme 2.3