正在加载图片...
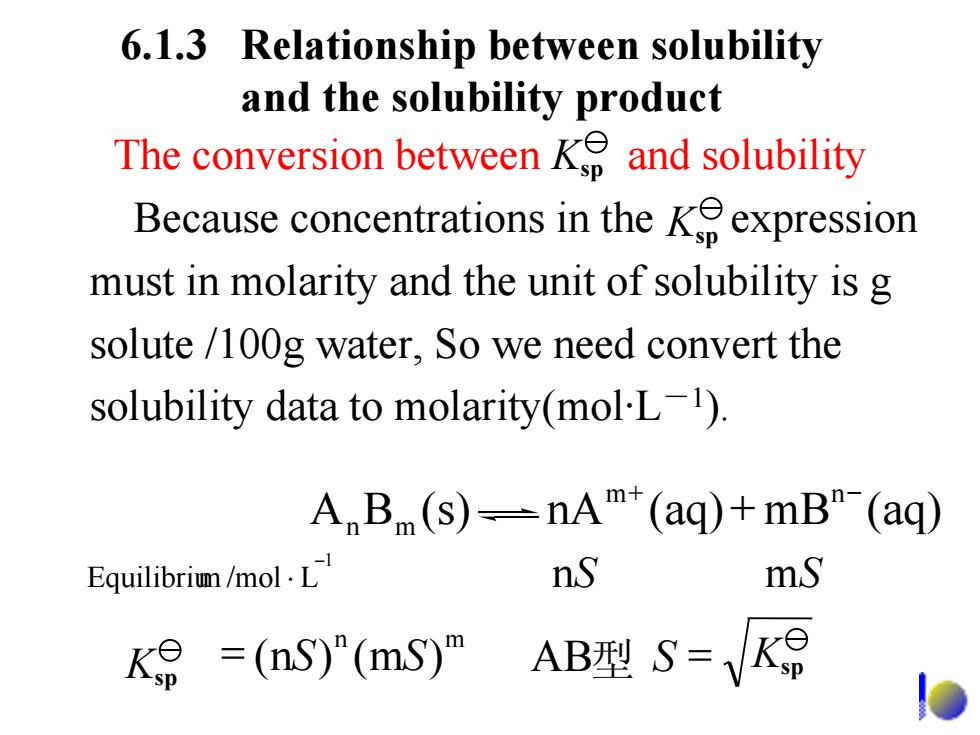
6.1.3 Relationship between solubility and the solubility product The conversion between K and solubility Because concentrations in theKeexpression must in molarity and the unit of solubility is g solute /100g water,So we need convert the solubility data to molarity(mol-L). A B (s)--nA*(aq)+mB"-(ag) Equilibrim /mol.L nS mS K=(nS)"(mS)"ABS= 6.1.3 Relationship between solubility and the solubility product 1 L /molmEquilibriu − ⋅ n S m S m + n − BA mn (s) nA (aq) + mB (aq) n m K = SS )m()n( sp AB 型 S = Ksp The conversion between and solubility Because concentrations in the expression must in molarity and the unit of solubility is g solute /100g water, So we need convert the solubility data to molarity(mol·L - 1). Ksp Ksp