正在加载图片...
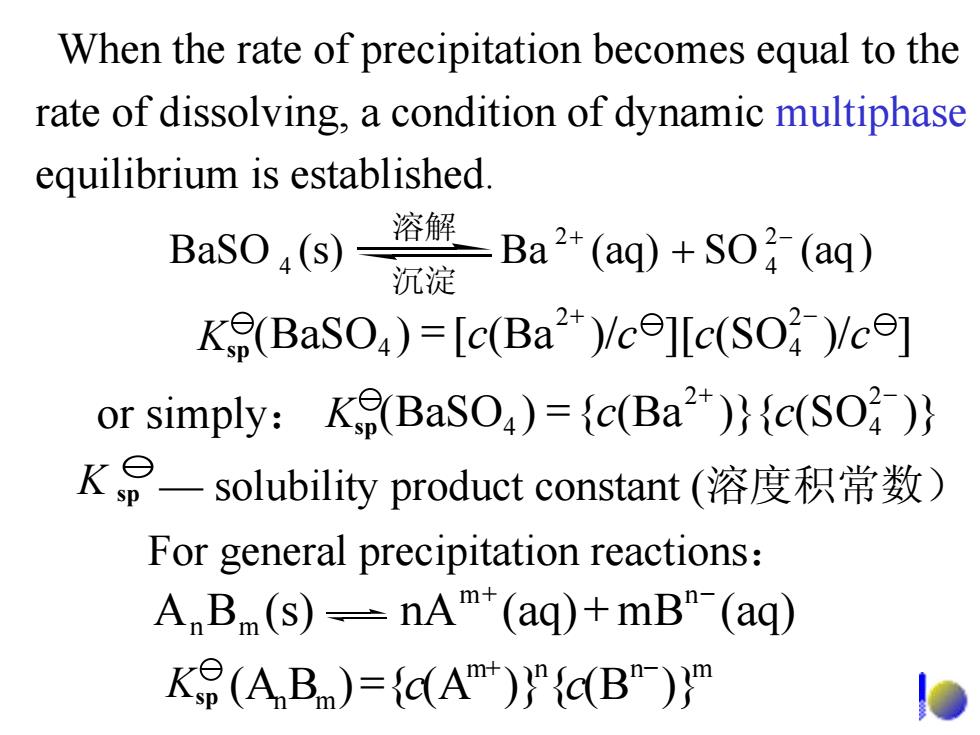
When the rate of precipitation becomes equal to the rate of dissolving,a condition of dynamic multiphase equilibrium is established. BaSO(s)= 溶解 Ba2*(aq)+SO(aq) 沉淀 K(BaSO)=[c(Ba2)/ce]lc(SO )/ce] or simply:K(BaSO)=c(Ba)(c(SO)) KP一solubility product constant(溶度积常数) For general precipitation reactions: A B(s)-nAm*(aq)+mB"(aq) K9(ABm)={C(Am)}”{c(B")}"When the rate of precipitation becomes equal to the rate of dissolving, a condition of dynamic multiphase equilibrium is established. For general precipitation reactions : K sp — solubility product constant (溶度积常数) BA (s) nA (aq) mB (aq) m n mn + − + mnnm mn (A {)B (A )} { (B )} + − Ksp = cc (BaSO (Ba{) )}{ (SO )} 2 4 2 4 + − or simply : Ksp = cc (BaSO (Ba[) ][)/ (SO )/ ] 2 4 2 4 + − Ksp = cccc 溶解 )(aqSO(aq)Ba (s)BaSO 2 4 2 4 + − + 沉淀