正在加载图片...
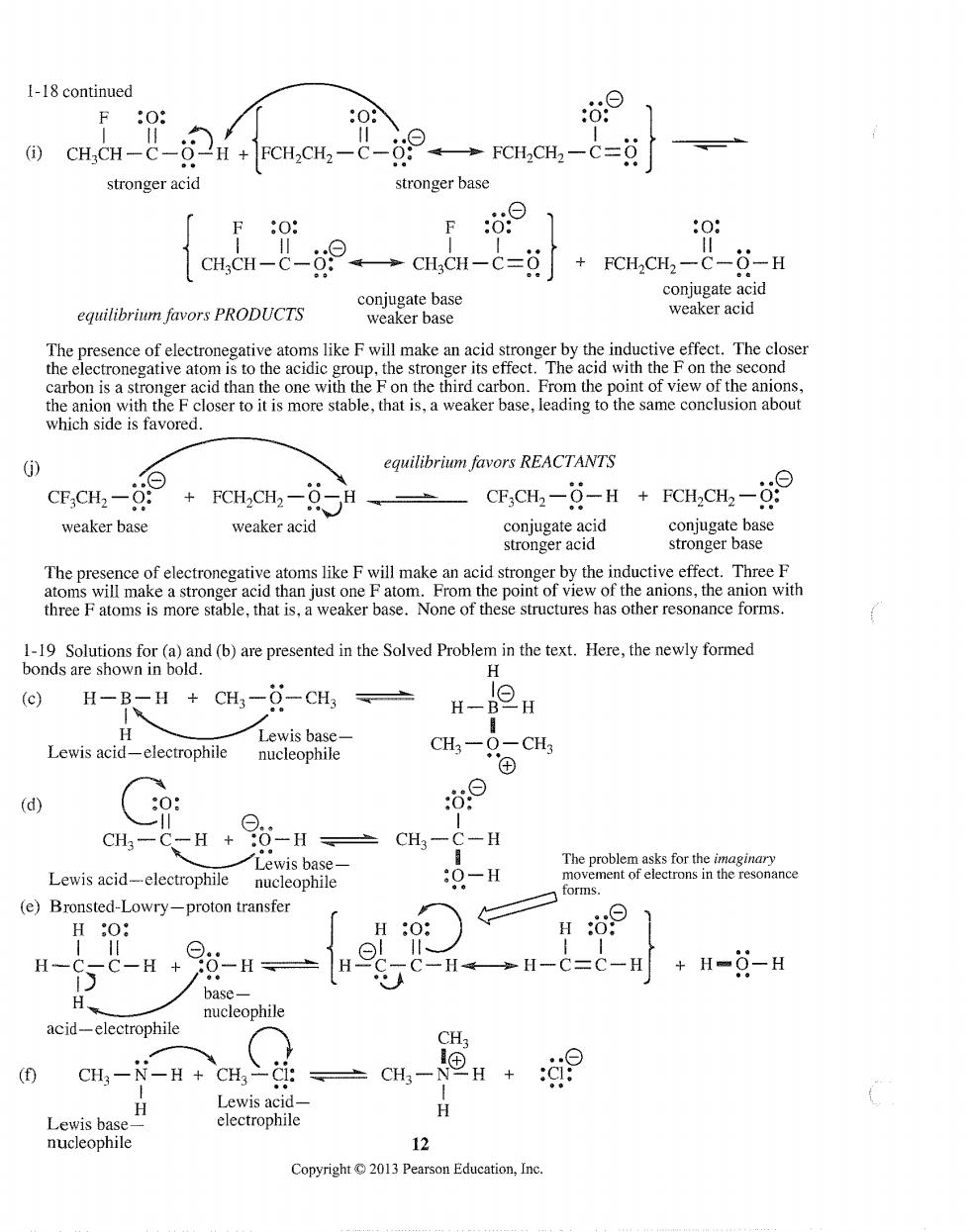
1-18 continued F:O: 08 :69 CH;CH-C- 2 H+FCH2CH2- →FCH,CH2-C=0i stronger acid stronger base F:O: F ⊙ cuen-2- 9。→cH,CH-c=9j FCHCH2-C-0-H equilibrium favors PRODUCTS tive ator s like f will make an acid st by the inductive effect.The closer m的 carbon is a equilibrium favors REACTANTS CF.CH.- FCH2CH2-6 G,CH-点-H+CHcH,-9 weaker base weaker acid coniugate acid conjugate base stronger acid stronger base The pre oms e anio with three atoms is more stable that is.weaker base.None of these stuctures has other resonance forms. 1-19 Solutions for(a)and(b)are presented in the Solved Problem in the text.Here,the newly formed bonds are shown in bold. H (c) H-B-H CH3-0-CH3 H-BOH H Lewis base- Lewis acid-electrophile nucleophile CH3-O-CH3 (d) 0 CH:-C-H +:0-H CH-( -H Lewis acid-electrophile nucleophile :O-H 、foms. (e)Bronsted-Lowry-proton transfer H0: H- -H+98-H H C--C=C-+H-0-H A acid-electrophile nucleophile CH; -N-H+CH3-C1: CH3 H+ 这9 Lewis acid- H Lewis has electrophile nucleophile 12 Copyright13 Pearson Education,Inc