正在加载图片...
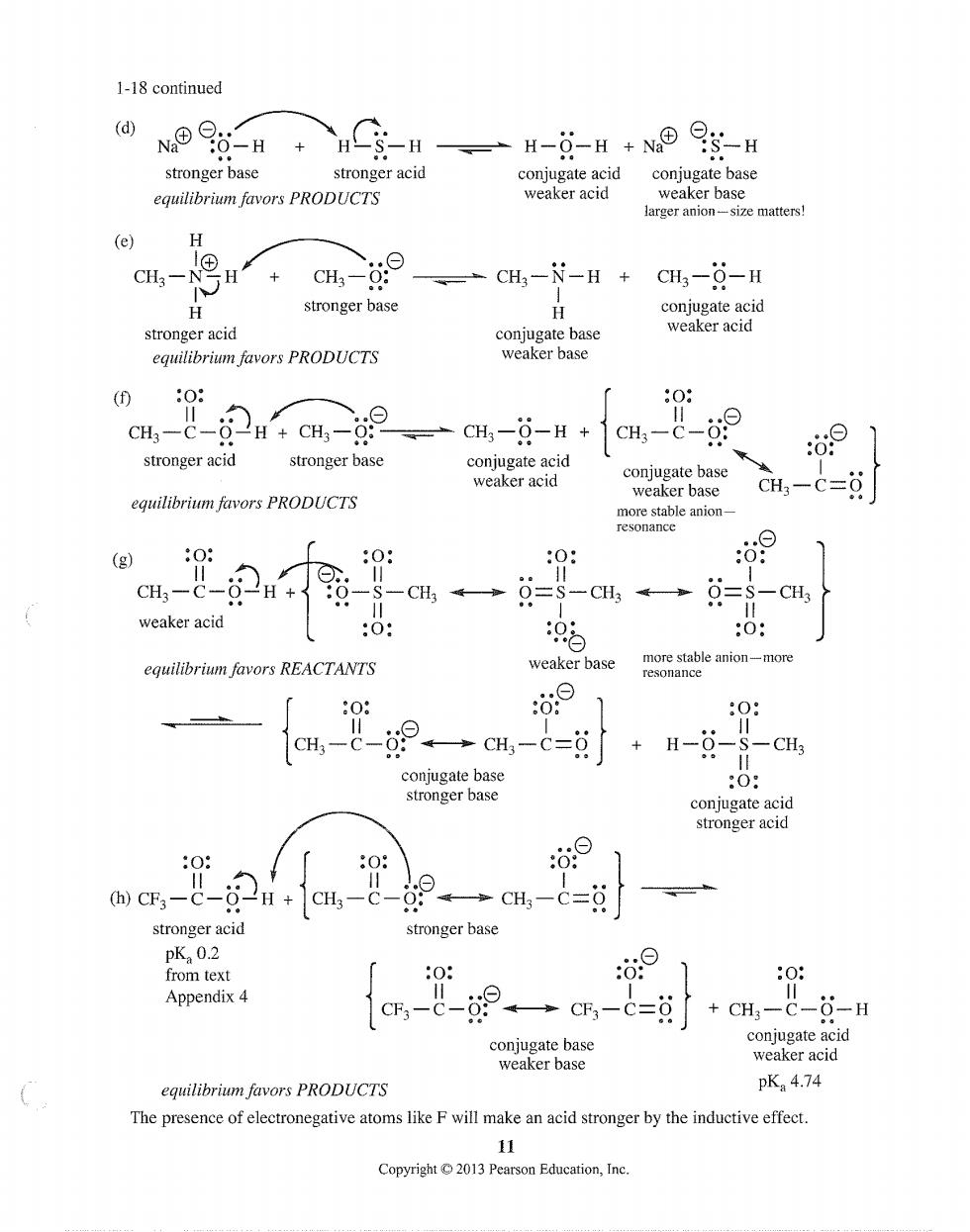
1-18 continued (d) N®a-H +C含-H一H-日-H+N0gH stronger base stronger acid conjugate acid conjugate base equilibrium favors PRODUCTS weaker acid ize matters H CH,- + CH3-0: 、=CH,-N-H+ CH3-6-H H stronger base H stronger acid conjugate base equilibrium favors PRODUCTS weaker base 08 0: H,-C-2HcHa9、一cH-g-H+ stronger acid conjugate acid -&-9 stronger base 0 weaker acid coniugate base equilibrium favors PRODUCTS weaker base CH-c=0 (g) :0: 0: :0: :d9 cH--246 -CH。→=s-CH→ =CH weaker acid :0: 05 :0: equilibrium favors REACTANTS weaker base le anion-more 0 00 1 : aH--89一cH-c=8+H-- -CH3 coniugate base stronger base :0: conjugate acid stronger acid 0 (h)CF-C- 2i+1em,- stronger acid stronger base pK0.2 from tex :0: ⊙ 0: Appendix 4 C,-C-→C,-C=g +CH-C-0-H conjugate base conjugate acid weaker base weaker acid equilibrium favors PRODUCTS pK:4.74 The presence of electronegative atoms like F will make an acid stronger by the inductive effect. Copyright C2013 Pearson Education,Inc