正在加载图片...
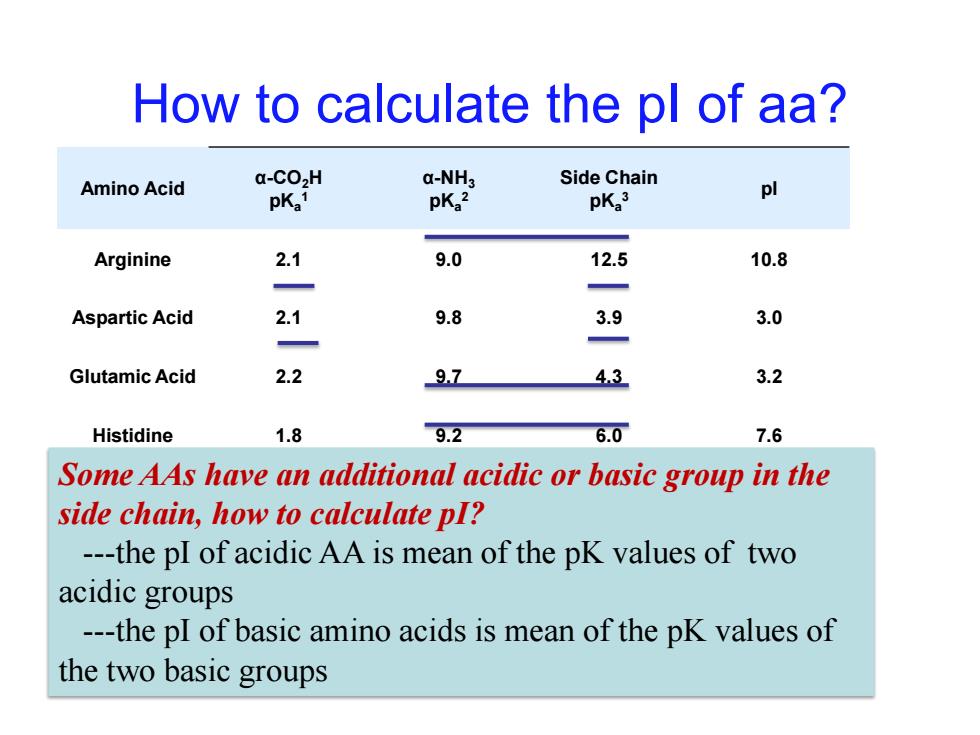
How to calculate the pl of aa? Amino Acid a-CO>H a-NH3 Side Chain pKa1 pKa2 pKa3 pl Arginine 2.1 9.0 12.5 10.8 Aspartic Acid 2.1 9.8 3.9 3.0 Glutamic Acid 2.2 97 43 3.2 Histidine 1.8 9.2 6.0 7.6 Some AAs have an additional acidic or basic group in the side chain,how to calculate pI? ---the pI of acidic AA is mean of the pK values of two acidic groups ---the pI of basic amino acids is mean of the pK values of the two basic groupsHow to calculate the pI of aa? Amino Acid α-CO2H pKa 1 α-NH3 pKa 2 Side Chain pKa 3 pI Arginine 2.1 9.0 12.5 10.8 Aspartic Acid 2.1 9.8 3.9 3.0 Glutamic Acid 2.2 9.7 4.3 3.2 Histidine 1.8 9.2 6.0 7.6 Some AAs have an additional acidic or basic group in the Lysine 2.2 9.0 10.5 9.8 side chain, how to calculate pI? ---the pI of acidic AA is mean of the pK values of two acidic groups ---the pI of basic amino acids is mean of the pK values of the two basic groups