CHAPTER 1 Proteins Overview Amino acid structures Four levels of organization in proteins -02 binding proteins:myoglobin and hemoglobin 2,3-Bisphosphoglycerate function in RBC protein physical-chemical properties and purifications methods Clinical correlations:Prion disease,Scurvy, Methemoglobinemia
CHAPTER 1 Proteins – Amino acid structures – Four levels of organization in proteins – O2 binding proteins: myoglobin and hemoglobin – 2,3-Bisphosphoglycerate function in RBC – protein physical-chemical properties and purifications methods – Clinical correlations: Prion disease, Scurvy, Methemoglobinemia Overview

Section Molecular Composition of Proteins life is maintained by molecular network system Protein make up about 15%of the cell Proteins play key roles in a living system Enzymes,Structural,Transport,Motor Storage,Signaling,Receptors,Gene regulation Special functions (antibody)
• life is maintained by molecular network system • Protein make up about 15% of the cell • Proteins play key roles in a living system Enzymes, Structural, Transport, Motor Storage, Signaling, Receptors, Gene regulation Special functions (antibody) ………. ……….. ………… Molecular Composition of Proteins Section 1
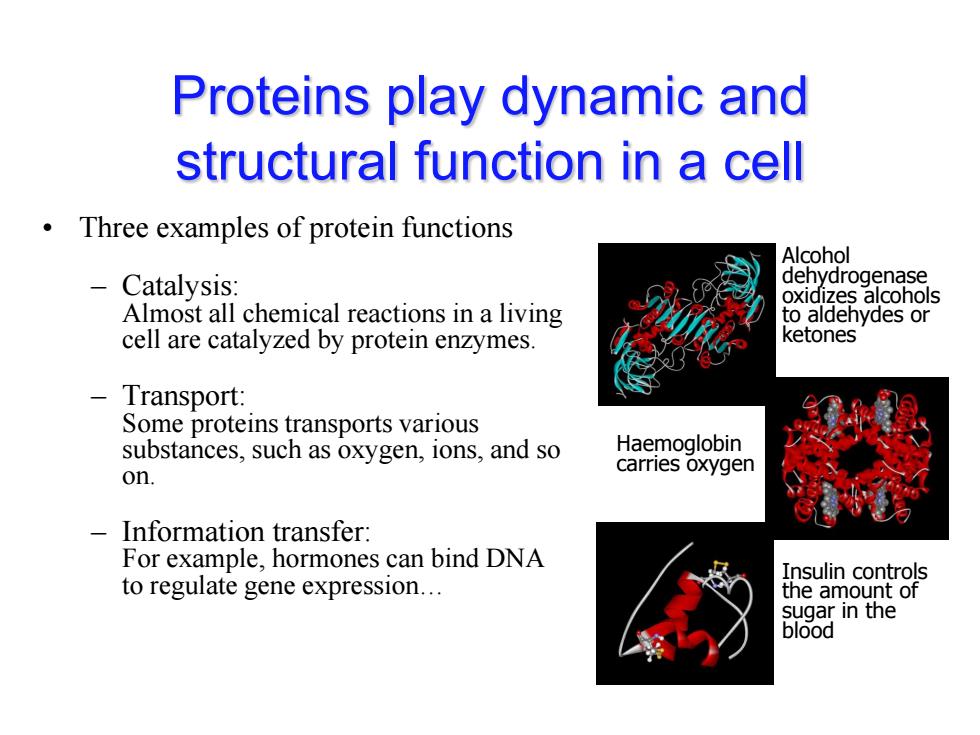
Proteins play dynamic and structural function in a cell Three examples of protein functions Alcohol Catalysis: dehydrogenase oxidizes alcohols Almost all chemical reactions in a living to aldehydes or cell are catalyzed by protein enzymes. ketones -Transport: Some proteins transports various substances,such as oxygen,ions,and so Haemoglobin on. carries oxygen Information transfer: For example,hormones can bind DNA Insulin controls to regulate gene expression... the amount of sugar in the blood
Proteins play dynamic and structural function in a cell • Three examples of protein functions – Catalysis: Almost all chemical reactions in a living cell are catalyzed by protein enzymes. – Transport: Some proteins transports various substances, such as oxygen, ions, and so on. – Information transfer: For example, hormones can bind DNA to regulate gene expression… Alcohol dehydrogenase oxidizes alcohols to aldehydes or ketones Haemoglobin carries oxygen Insulin controls the amount of sugar in the blood

Alcohol acts as a central nervous system depressant alcohol abuse

The Chemical Breakdown of Alcohol CH CH,OH_ADH>CHaCHO ALDH>CH COO- Acetaldehyde Acetate The chemical name for alcohol is ethanol(CH3CH2OH).The body processes and eliminates ethanol in separate steps.Chemicals called enzymes help to break apart the ethanol molecule into other compounds (or metabolites),which can be processed more easily by the body.Some of these intermediate metabolites can have harmful effects on the body. Most of the ethanol in the body is broken down in the liver by an enzyme called alcohol dehydrogenase (ADH),which transforms ethanol into a toxic compound called acetaldehyde(CH3CHO),a known carcinogen.However,acetaldehyde is generally short-lived; it is quickly broken down to a less toxic compound called acetate (CHaCOO)by another enzyme called aldehyde dehydrogenase (ALDH).Acetate then is broken down to carbon dioxide and water. mainly in tissues other than the liver
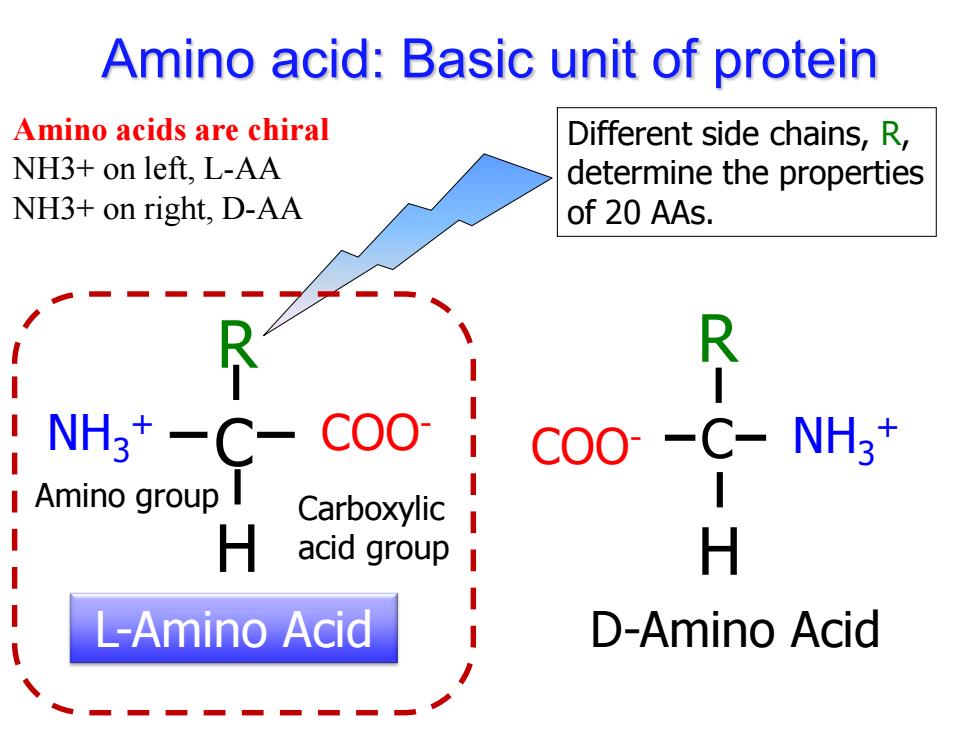
Amino acid:Basic unit of protein Amino acids are chiral Different side chains,R, NH3+on left.L-AA determine the properties NH3+on right,D-AA of 20 AAs. C- NH虜*一C- C00 COO--C-NH3+ I Amino group I Carboxylic H acid group H L-Amino Acid D-Amino Acid
Amino acid: Basic unit of protein NH COO- 3 + C R H L-Amino Acid Different side chains, R, determine the properties of 20 AAs. Amino group Carboxylic acid group Amino acids are chiral NH3+ on left, L-AA NH3+ on right, D-AA COO- NH3 + C R H D-Amino Acid

Amino acids are zwitterion The protonation state of alanine pk of COO-close to 2.34 pk of NH3+near 9.69 Alanine 14.0 12.0h pK2=9.69 10.0 H2N-H CH3 8.0 8.0- pH>10 pH9 02 6.0 =601 6.0- HgN-H cation?anion? 4.0 4.0 CH3 pH between 2 to 9? pKg1=2.34 pH≈6 2.0 2.0 9C02H H3N-H CH3 0.5 1.0 1.5 2.0 2.5 <2 Equivalents of OH" pl=1/2(pkaCOOH+pkaNH3+) The zwitterion of alanine is the predominant form in the pH range from 2.3 to 9.9
Amino acids are zwitterion The zwitterion of alanine is the predominant form in the pH range from 2.3 to 9.9 pk of COO- close to 2.34 pk of NH3+ near 9.69 pH 9 cation? anion? pH between 2 to 9? The protonation state of alanine pI=1/2(pkaCOOH+pkaNH3+)
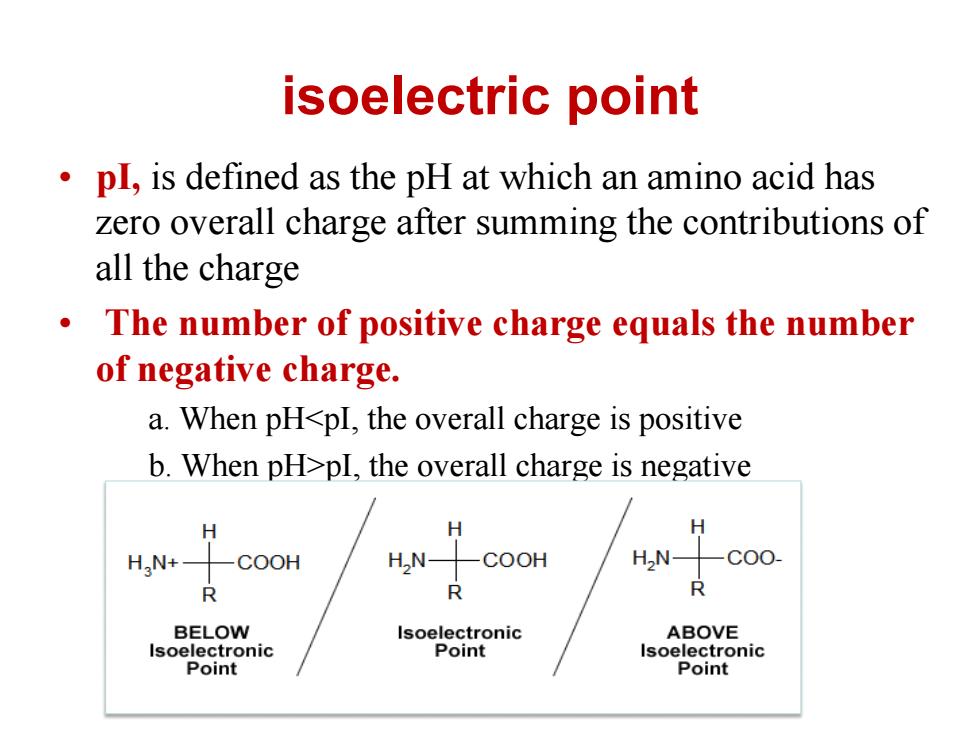
isoelectric point pl,is defined as the pH at which an amino acid has zero overall charge after summing the contributions of all the charge 。 The number of positive charge equals the number of negative charge. a.When pHpI,the overall charge is negative H HaN+- COOH H,N COOH HN- -C00- R R R BELOW Isoelectronic ABOVE Isoelectronic Point Isoelectronic Point Point
isoelectric point • pI, is defined as the pH at which an amino acid has zero overall charge after summing the contributions of all the charge • The number of positive charge equals the number of negative charge. a. When pHpI, the overall charge is negative c. When pH=pI, there is no overall charge
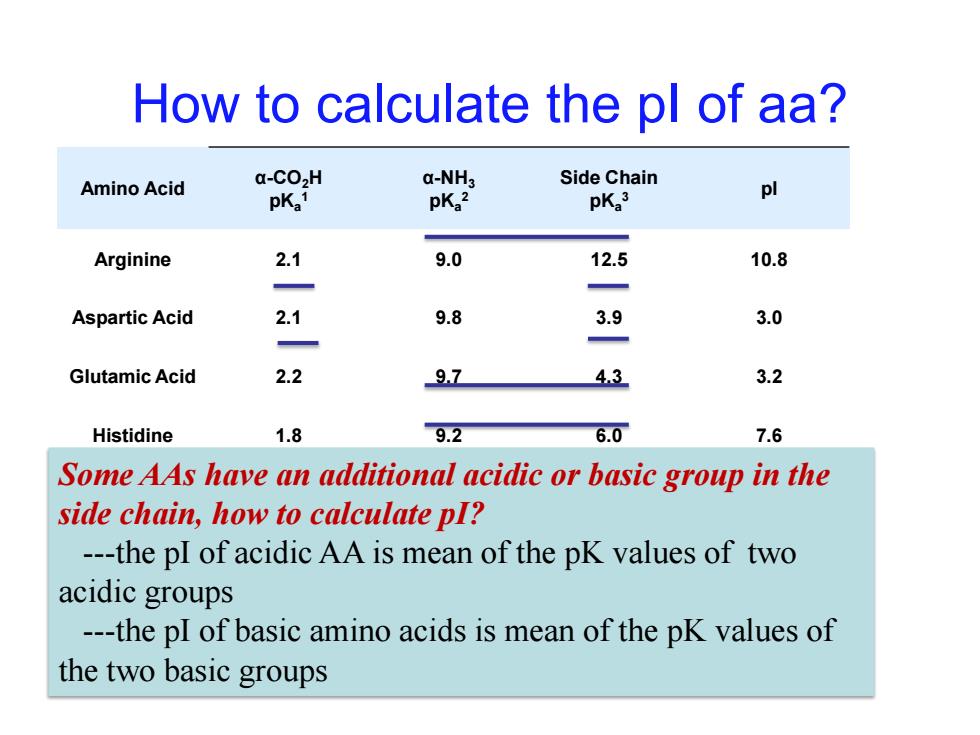
How to calculate the pl of aa? Amino Acid a-CO>H a-NH3 Side Chain pKa1 pKa2 pKa3 pl Arginine 2.1 9.0 12.5 10.8 Aspartic Acid 2.1 9.8 3.9 3.0 Glutamic Acid 2.2 97 43 3.2 Histidine 1.8 9.2 6.0 7.6 Some AAs have an additional acidic or basic group in the side chain,how to calculate pI? ---the pI of acidic AA is mean of the pK values of two acidic groups ---the pI of basic amino acids is mean of the pK values of the two basic groups
How to calculate the pI of aa? Amino Acid α-CO2H pKa 1 α-NH3 pKa 2 Side Chain pKa 3 pI Arginine 2.1 9.0 12.5 10.8 Aspartic Acid 2.1 9.8 3.9 3.0 Glutamic Acid 2.2 9.7 4.3 3.2 Histidine 1.8 9.2 6.0 7.6 Some AAs have an additional acidic or basic group in the Lysine 2.2 9.0 10.5 9.8 side chain, how to calculate pI? ---the pI of acidic AA is mean of the pK values of two acidic groups ---the pI of basic amino acids is mean of the pK values of the two basic groups

What's the significance of zwitterion property? Ionizable groups stabilize the pH value of the solution against external disturbances. Any ionizable group buffers the pH of the solution at pH value close to its pK. The buffering capacity of ionizable groups helps human maintain a constant in body fluid. A contribution to homeostatsis
What’s the significance of zwitterion property? • Ionizable groups stabilize the pH value of the solution against external disturbances. • Any ionizable group buffers the pH of the solution at pH value close to its pK. • The buffering capacity of ionizable groups helps human maintain a constant in body fluid. A contribution to homeostatsis