
What have you heard about enzymes? 2
What have you heard about enzymes? 2
Think about your breakfast If eaten,digested in hours; If not eaten,stay for years..... 3
Think about your breakfast If eaten, digested in hours; If not eaten, stay for years….. 3
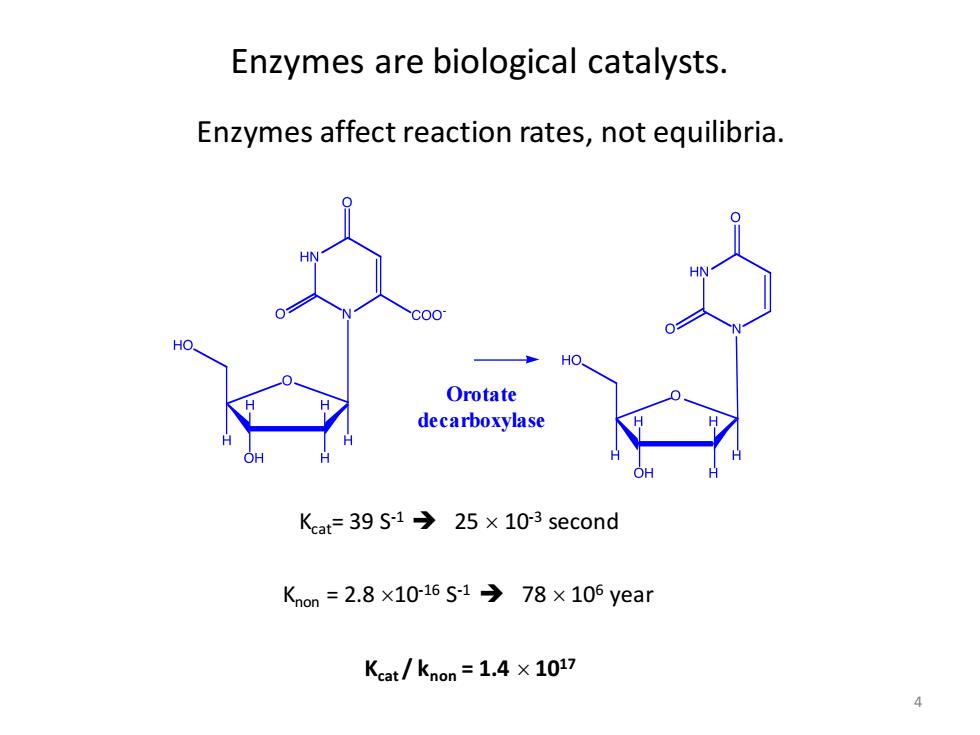
Enzymes are biological catalysts Enzymes affect reaction rates,not equilibria. HO Orotate decarboxylase Kat=39S-1→25×103 second Kon=2.8×1016S-1→78×105year Kcat/knon=1.4×1017 4
4 Orotate decarboxylase Knon = 2.8 10-16 S -1 ➔ 78 106 year Kcat= 39 S-1 ➔ 25 10-3 second Kcat / knon = 1.4 1017 Enzymes are biological catalysts. Enzymes affect reaction rates, not equilibria
Most enzymes are proteins. Could be monomeric or oligomeric enzymes; Could be simple or conjugated enzymes; holoenzyme=apoenzyme cofactor 可 Table 3.1 for the most important coenzymes. 5
Most enzymes are proteins. Could be monomeric or oligomeric enzymes; Could be simple or conjugated enzymes; holoenzyme = apoenzyme + cofactor Table 3.1 for the most important coenzymes. 5
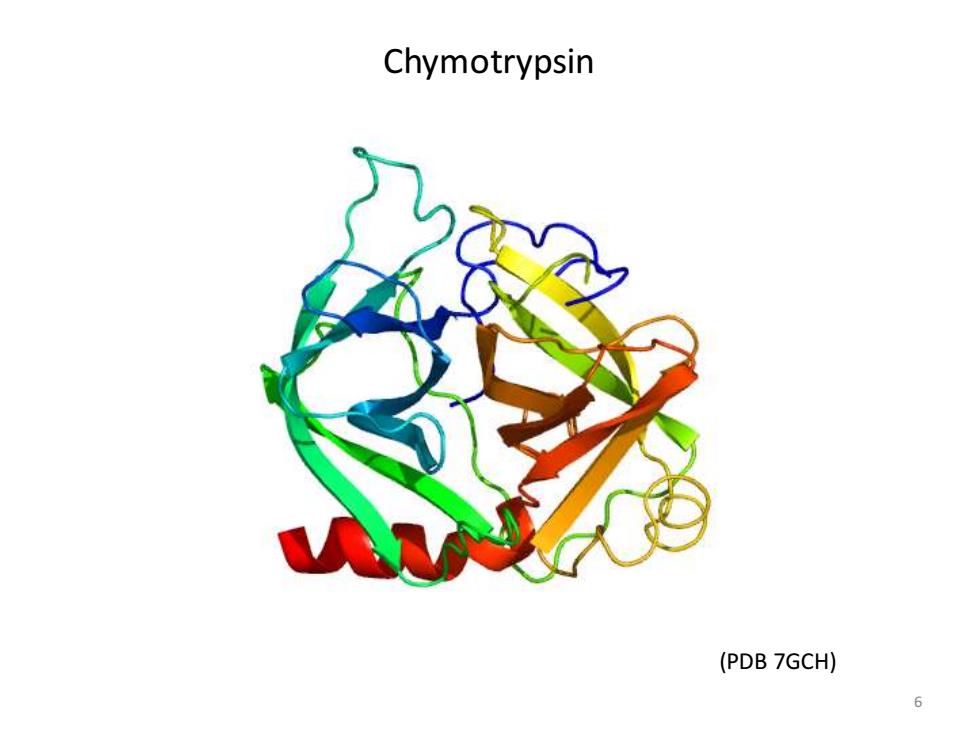
Chymotrypsin (PDB 7GCH)
6 Chymotrypsin (PDB 7GCH)

Chymotrypsin Active site 7
7 Chymotrypsin Active site
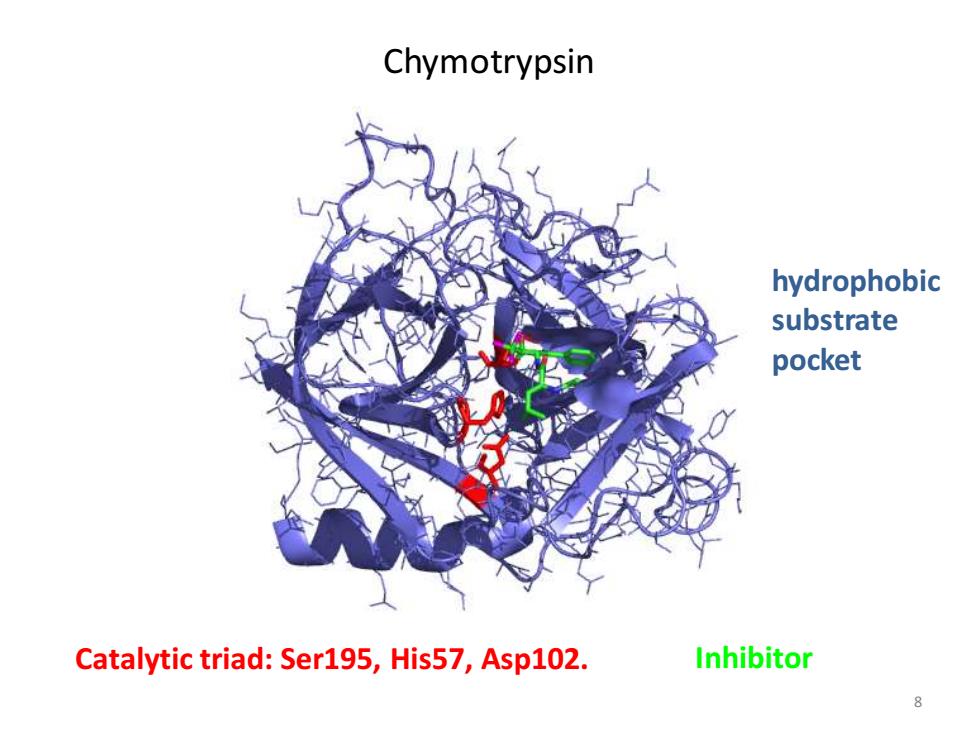
Chymotrypsin hydrophobic substrate pocket Catalytic triad:Ser195,His57,Asp102. Inhibitor
8 Catalytic triad: Ser195, His57, Asp102. Inhibitor Chymotrypsin hydrophobic substrate pocket
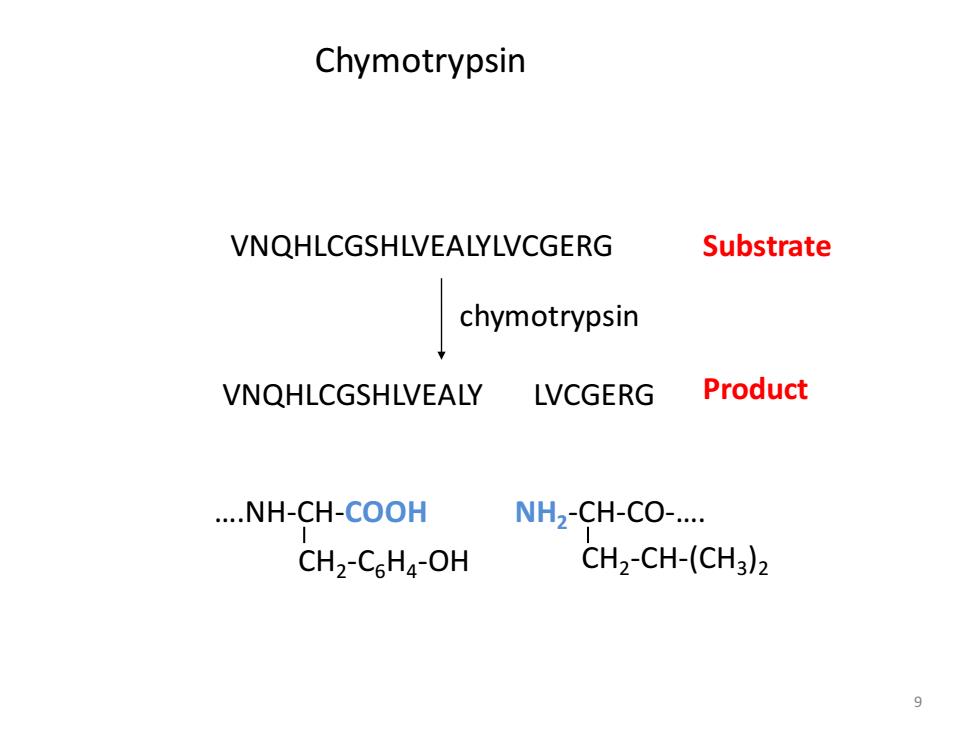
Chymotrypsin VNQHLCGSHLVEALYLVCGERG Substrate chymotrypsin VNQHLCGSHLVEALY LVCGERG Product ....NH-CH-COOH NH2-CH-CO-.... CH2-CsHa-OH CH2-CH-(CH3)2 9
9 VNQHLCGSHLVEALYLVCGERG VNQHLCGSHLVEALY LVCGERG chymotrypsin ….NH-CH-COOH CH2 -C6H4 -OH NH2 -CH-CO-…. CH2 -CH-(CH3 )2 Substrate Product Chymotrypsin

Mechanism of chymotrypsin (juang.bst.ntu.edu.tw/BCbasics/Animation1.htm) 10
10 Mechanism of chymotrypsin (juang.bst.ntu.edu.tw/BCbasics/Animation1.htm)
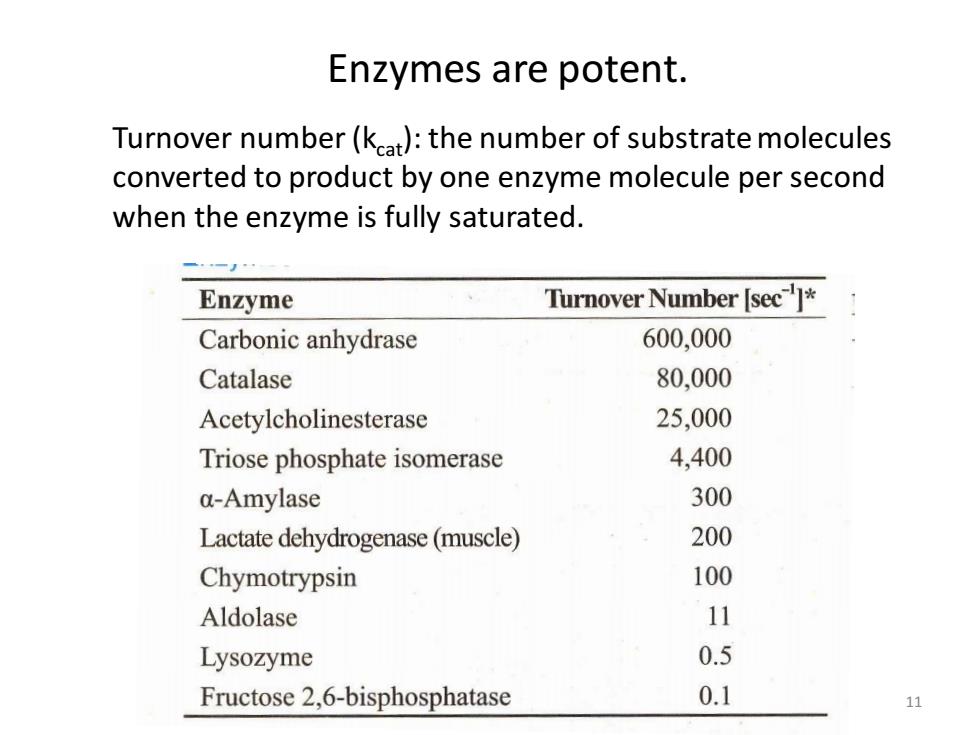
Enzymes are potent. Turnover number(kat):the number of substrate molecules converted to product by one enzyme molecule per second when the enzyme is fully saturated. Enzyme Turnover Number [sec]* Carbonic anhydrase 600,000 Catalase 80,000 Acetylcholinesterase 25,000 Triose phosphate isomerase 4,400 a-Amylase 300 Lactate dehydrogenase(muscle) 200 Chymotrypsin 100 Aldolase 11 Lysozyme 0.5 Fructose 2,6-bisphosphatase 0.1 11
Enzymes are potent. 11 Turnover number (kcat): the number of substrate molecules converted to product by one enzyme molecule per second when the enzyme is fully saturated