
Aerobic Metabolism Il: Electron Transport and Oxidative Phosphorylation
Aerobic Metabolism II: Electron Transport and Oxidative Phosphorylation

-the use of oxygen by aerobes is of great advantage as it provides much more energy than anaerobic fermentation -used by aerobic organisms as a terminal electron acceptor in energy generation
O2 -the use of oxygen by aerobes is of great advantage as it provides much more energy than anaerobic fermentation -used by aerobic organisms as a terminal electron acceptor in energy generation

O2 (cont.) it is suitable for this role because: it is readily available (occurs almost everywhere on the earth) it diffuses easily across cell membranes it is highly reactive and thus readily accepts electrons the use of O,by aerobic organisms is linked to the production of highly destructive ROS (reactive oxygen species)such as:hydrogen peroxide, superoxide radical,hydroxy radical,and singlet oxygen
- it is suitable for this role because: * it is readily available (occurs almost everywhere on the earth) * it diffuses easily across cell membranes * it is highly reactive and thus readily accepts electrons - the use of O2 by aerobic organisms is linked to the production of highly destructive ROS (reactive oxygen species) such as: hydrogen peroxide, superoxide radical, hydroxy radical, and singlet oxygen O2 (cont.)
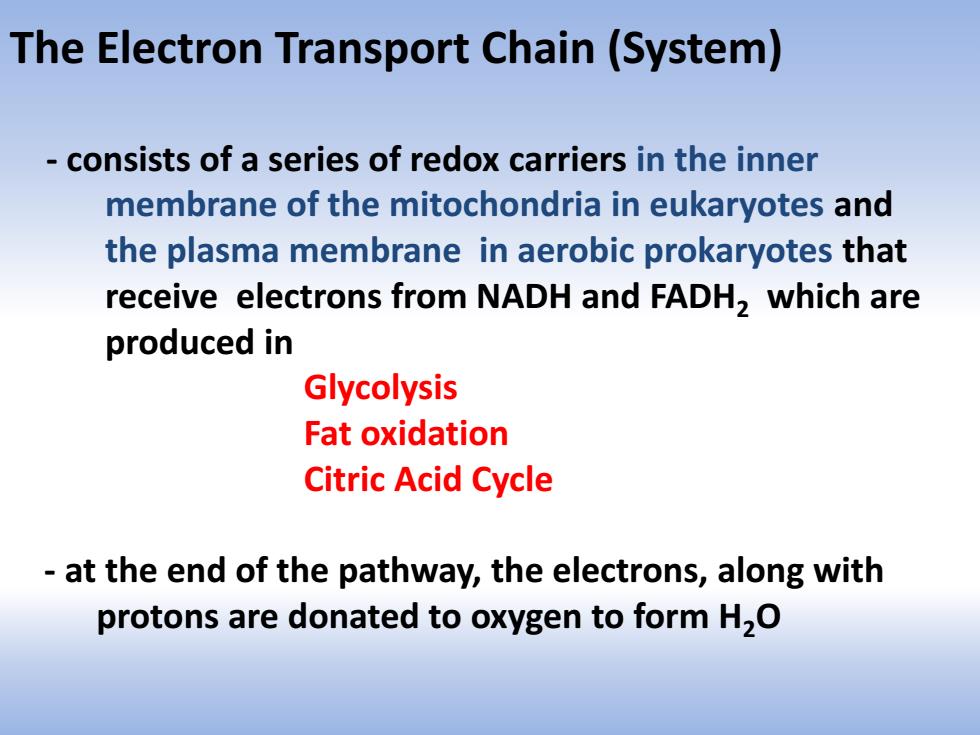
The Electron Transport Chain(System) consists of a series of redox carriers in the inner membrane of the mitochondria in eukaryotes and the plasma membrane in aerobic prokaryotes that receive electrons from NADH and FADH,which are produced in Glycolysis Fat oxidation Citric Acid Cycle at the end of the pathway,the electrons,along with protons are donated to oxygen to form H,O
The Electron Transport Chain (System) - consists of a series of redox carriers in the inner membrane of the mitochondria in eukaryotes and the plasma membrane in aerobic prokaryotes that receive electrons from NADH and FADH2 which are produced in Glycolysis Fat oxidation Citric Acid Cycle - at the end of the pathway, the electrons, along with protons are donated to oxygen to form H2O
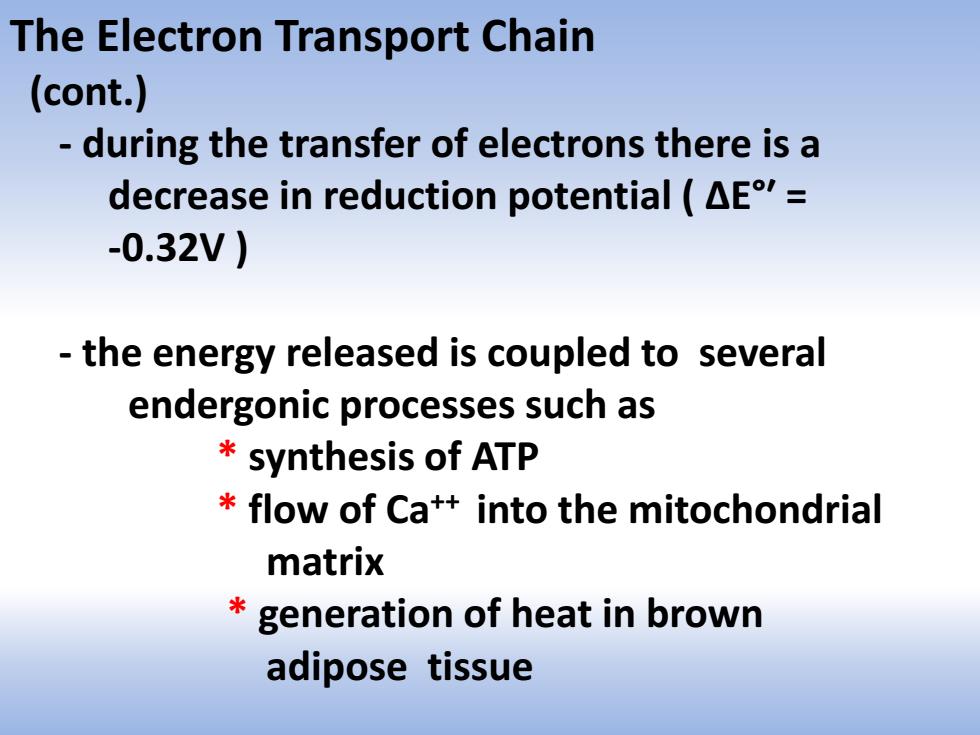
The Electron Transport Chain (cont.) during the transfer of electrons there is a decrease in reduction potential (AE= -0.32V) the energy released is coupled to several endergonic processes such as synthesis of ATP flow of Ca++into the mitochondrial matrix generation of heat in brown adipose tissue
The Electron Transport Chain (cont.) - during the transfer of electrons there is a decrease in reduction potential ( ΔE°ʹ = -0.32V ) - the energy released is coupled to several endergonic processes such as * synthesis of ATP * flow of Ca++ into the mitochondrial matrix * generation of heat in brown adipose tissue
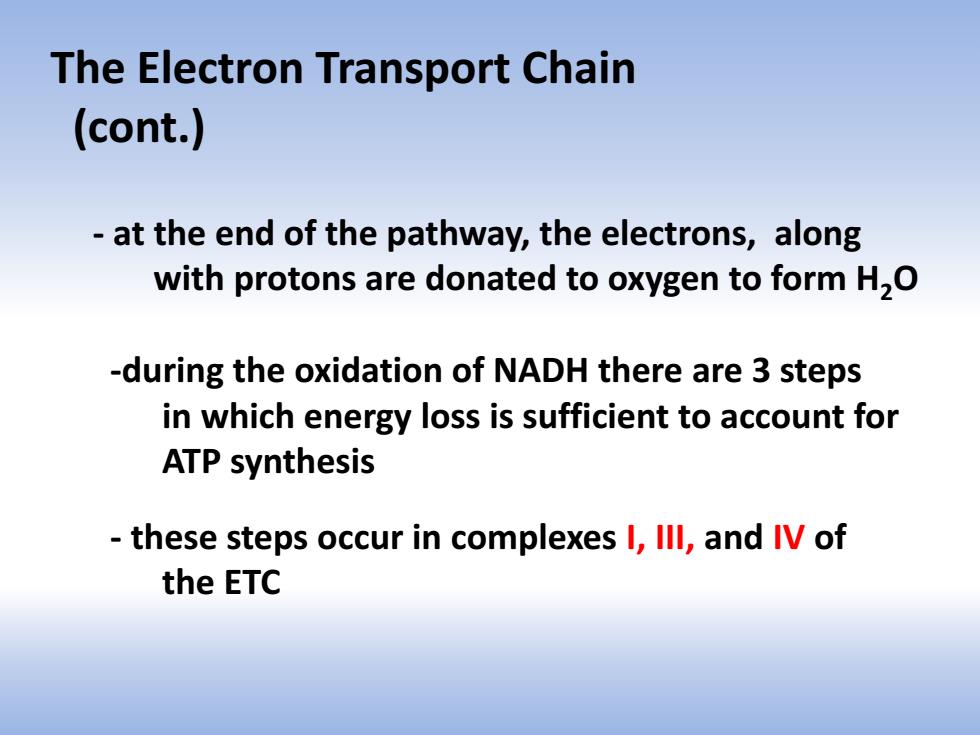
The Electron Transport Chain (cont.) at the end of the pathway,the electrons,along with protons are donated to oxygen to form H,O -during the oxidation of NADH there are 3 steps in which energy loss is sufficient to account for ATP synthesis these steps occur in complexes I,Ill,and IV of the ETC
- at the end of the pathway, the electrons, along with protons are donated to oxygen to form H2O -during the oxidation of NADH there are 3 steps in which energy loss is sufficient to account for ATP synthesis - these steps occur in complexes I, III, and IV of the ETC The Electron Transport Chain (cont.)
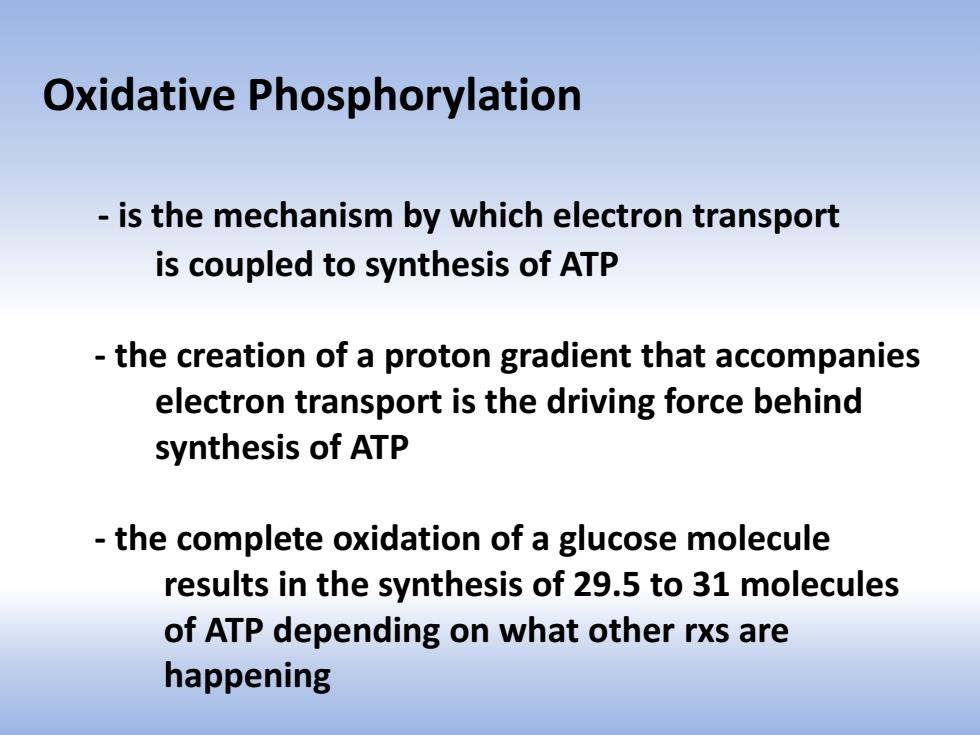
Oxidative Phosphorylation is the mechanism by which electron transport is coupled to synthesis of ATP the creation of a proton gradient that accompanies electron transport is the driving force behind synthesis of ATP the complete oxidation of a glucose molecule results in the synthesis of 29.5 to 31 molecules of ATP depending on what other rxs are happening
Oxidative Phosphorylation - is the mechanism by which electron transport is coupled to synthesis of ATP - the creation of a proton gradient that accompanies electron transport is the driving force behind synthesis of ATP - the complete oxidation of a glucose molecule results in the synthesis of 29.5 to 31 molecules of ATP depending on what other rxs are happening
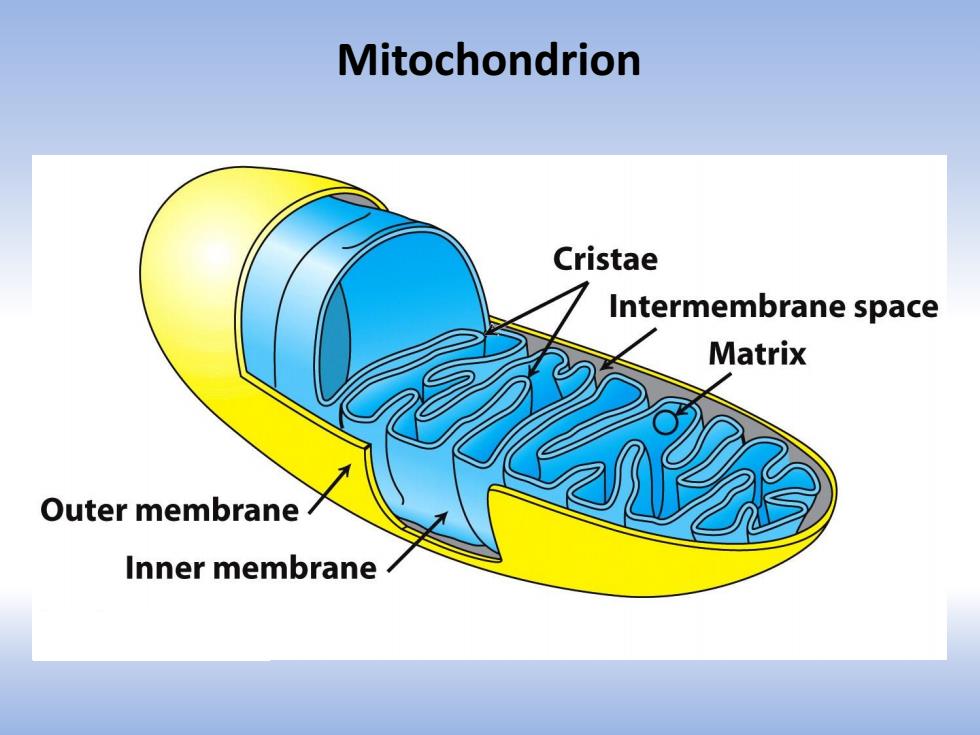
Mitochondrion Cristae Intermembrane space Matrix Outer membrane Inner membrane
Mitochondrion
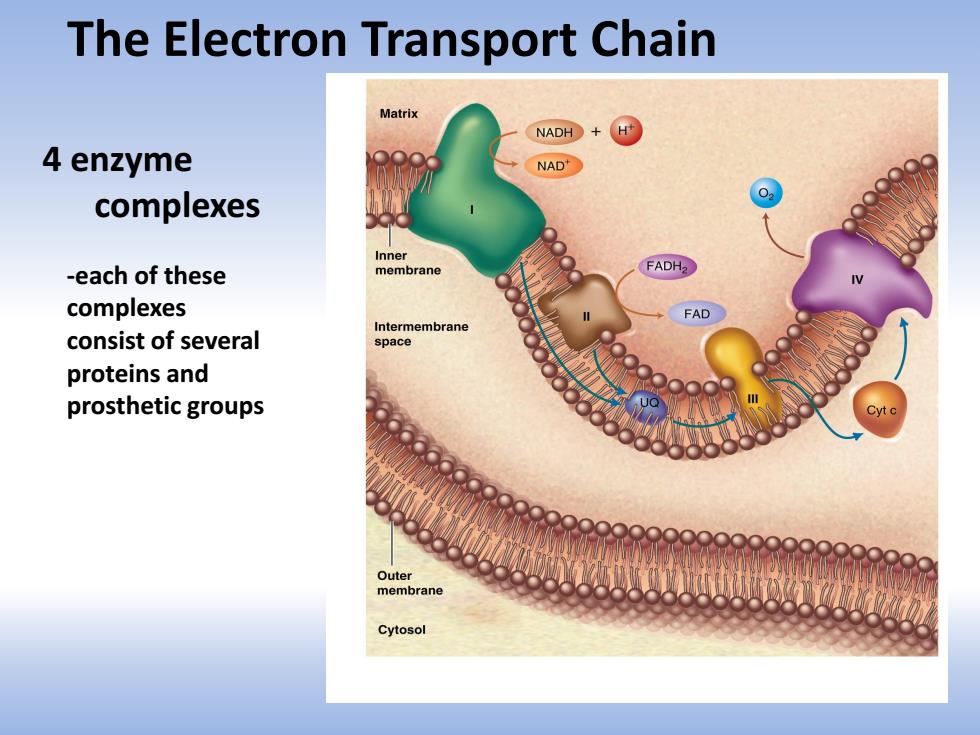
The Electron Transport Chain Matrix NADH+H 4 enzyme NAD 02 complexes Inner -each of these membrane FADH2 么 complexes FAD Intermembrane consist of several space proteins and prosthetic groups Outer membrane Cytosol
The Electron Transport Chain 4 enzyme complexes -each of these complexes consist of several proteins and prosthetic groups
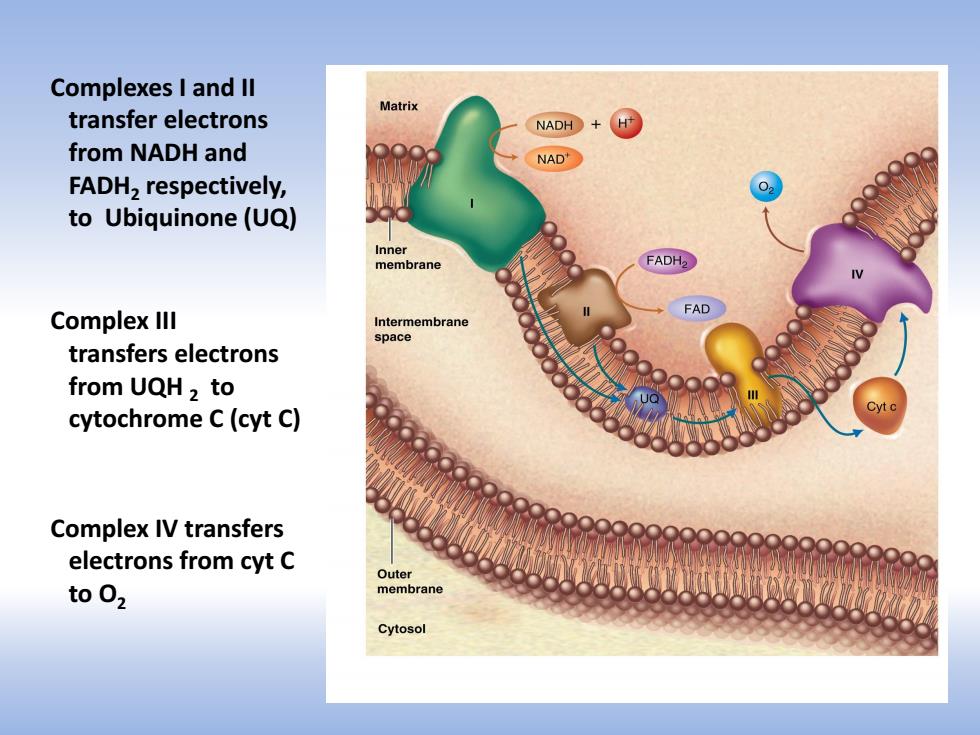
Complexes I and Il Matrix transfer electrons NADH+ from NADH and NAD' FADH2 respectively, to Ubiquinone(UQ) Inner membrane FADH2 么 FAD Complex Ill Intermembrane space transfers electrons from UQH2 to cytochrome C(cyt C) Complex IV transfers electrons from cyt C Outer toO2 membrane Cytosol
Complexes I and II transfer electrons from NADH and FADH2 respectively, to Ubiquinone (UQ) Complex III transfers electrons from UQH 2 to cytochrome C (cyt C) Complex IV transfers electrons from cyt C to O2