
CHAPTER 4 Mass Transport in Bioreactor
Chapter 4 Mass Transport in Bioreactor O2 O2
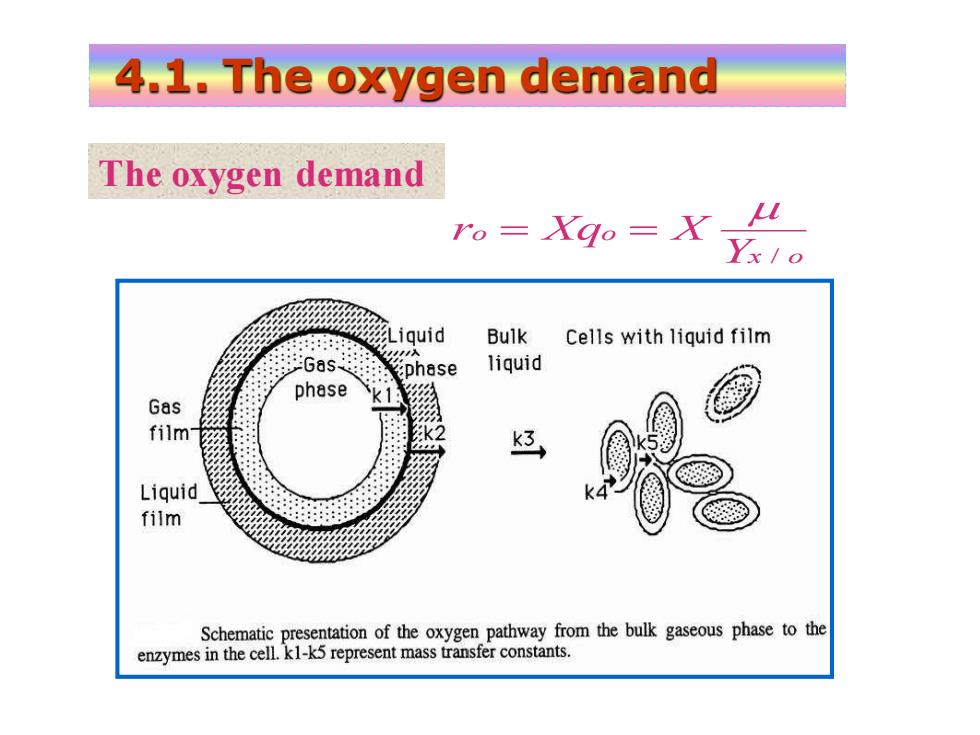
4.1.The oxygen demand The oxygen demand ro=Xgo=X Liquid Bulk Cells with liquid film :Gas: phase liquid phase Gas film Liquid film Schematic presentation of the oxygen pathway from the bulk gaseous phase to the enzymes in the cell.kl-k5 represent mass transfer constants
x o o o Y r Xq X / = = 4.1. The oxygen demand The oxygen demand

4.2.The oxygen transport Gas phase Liquid film Bulk liquid C=C米 0<C<C¥ C=0 一6 Distance Oxygen concentration gradients at the gas-liquid interface.Cg is the oxygen concentration in the gas phase(about 300 mg/L).At the liquid side of the interface(=0)the dissolved oxygen concentration C*is given by Henry's law.It is about 7 mg/L in water in equilibrium with air.Depending on the oxygen consumption rate in the liquid different concentration gradients(a-d)will appear through the liquid film with the thickness
4.2. The oxygen transport
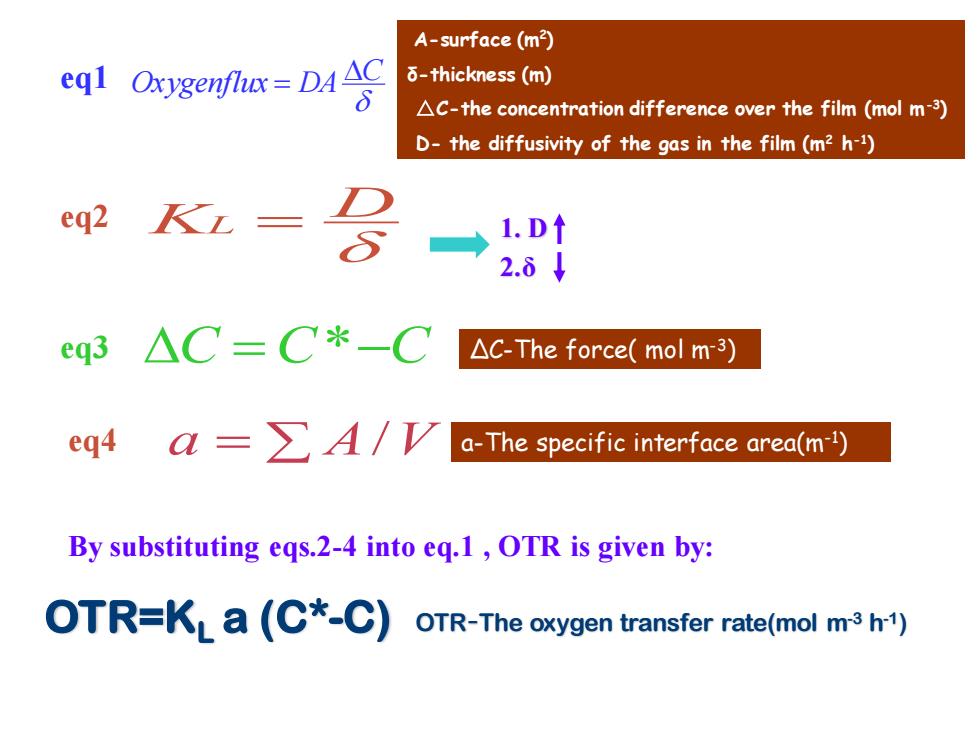
A-surface (m2) eql Oxygenfte-DA 6-thickness(m) AC-the concentration difference over the film(mol m-3) D-the diffusivity of the gas in the film(m2 h-1) eq2 KL- 2.8↓ eq3 △C=C*-C △c-The force(molm3) eq4a=∑A/V a-The specific interface area(m-) By substituting eqs.2-4 into eq.1,OTR is given by: OTR=KLa(C*-C)OTR-The oxygen transfer rate(mol m3h1)
OTR-The oxygen transfer rate(mol m-3 h-1) KL = D C =C*−C a = A/V A-surface (m2 ) δ-thickness (m) △C-the concentration difference over the film (mol m-3 ) D- the diffusivity of the gas in the film (m2 h -1 ) OTR=KL a (C*-C) 1. D 2.δ C Oxygenflux DA = ΔC-The force( mol m-3) a-The specific interface area(m-1 ) By substituting eqs.2-4 into eq.1 , OTR is given by: eq1 eq2 eq3 eq4

OTR=K4C-C K air,stirrer and speed,viscosity ◆A small gas bubble ◆ C* oxygen carrier temperature and pressure ◆C recom binent DNA
OTR=KL a (C*-C) ◆ KL air, stirrer and speed, viscosity ◆ A small gas bubble ◆ C* oxygen carrier temperature and pressure ◆C recombinent DNA
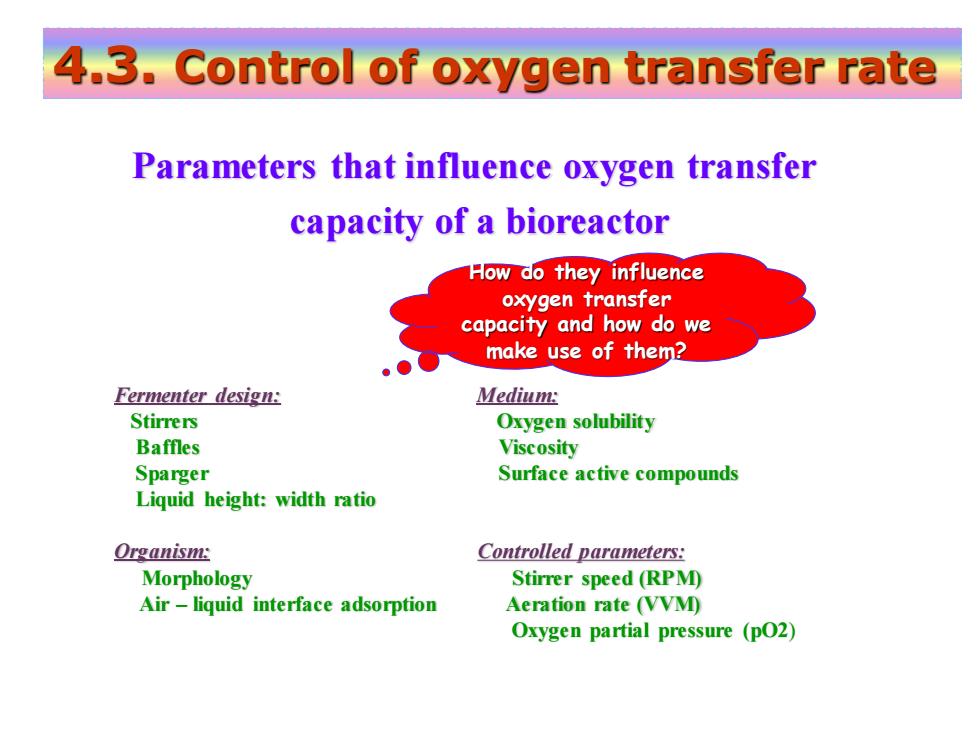
4.3.Control of oxygen transfer rate Parameters that influence oxygen transfer capacity of a bioreactor How do they influence oxygen transfer capacity and how do we make use of them? Fermenter design: Medium: Stirrers Oxygen solubility Baffles Viscosity Sparger Surface active compounds Liquid height:width ratio Organism: Controlled parameters: Morphology Stirrer speed(RPM) Air-liquid interface adsorption Aeration rate (VVM) Oxygen partial pressure (pO2)
Parameters that influence oxygen transfer capacity of a bioreactor 4.3. Control of oxygen transfer rate Fermenter design: Medium: Stirrers Oxygen solubility Baffles Viscosity Sparger Surface active compounds Liquid height: width ratio Organism: Controlled parameters: Morphology Stirrer speed (RPM) Air – liquid interface adsorption Aeration rate (VVM) Oxygen partial pressure (pO2) How do they influence oxygen transfer capacity and how do we make use of them?
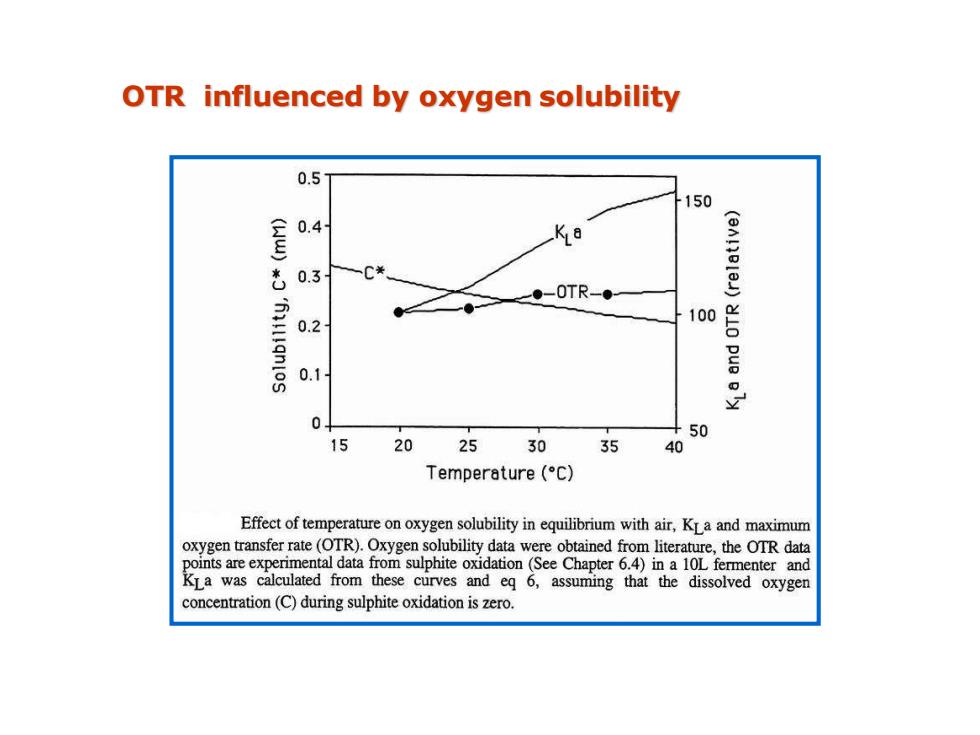
OTR influenced by oxygen solubility 0.5 150 0.4 0.3 C* ●-0TR-● 'llqnlos 0.2 100 0.1 器 50 15 20 2530 35 40 Temperature(C) Effect of temperature on oxygen solubility in equilibrium with air,KLa and maximum oxygen transfer rate(OTR).Oxygen solubility data were obtained from literature,the OTR data points are experimental data from sulphite oxidation(See Chapter 6.4)in a 10L fermenter and KLa was calculated from these curves and eq 6,assuming that the dissolved oxygen concentration(C)during sulphite oxidation is zero
OTR influenced by oxygen solubility
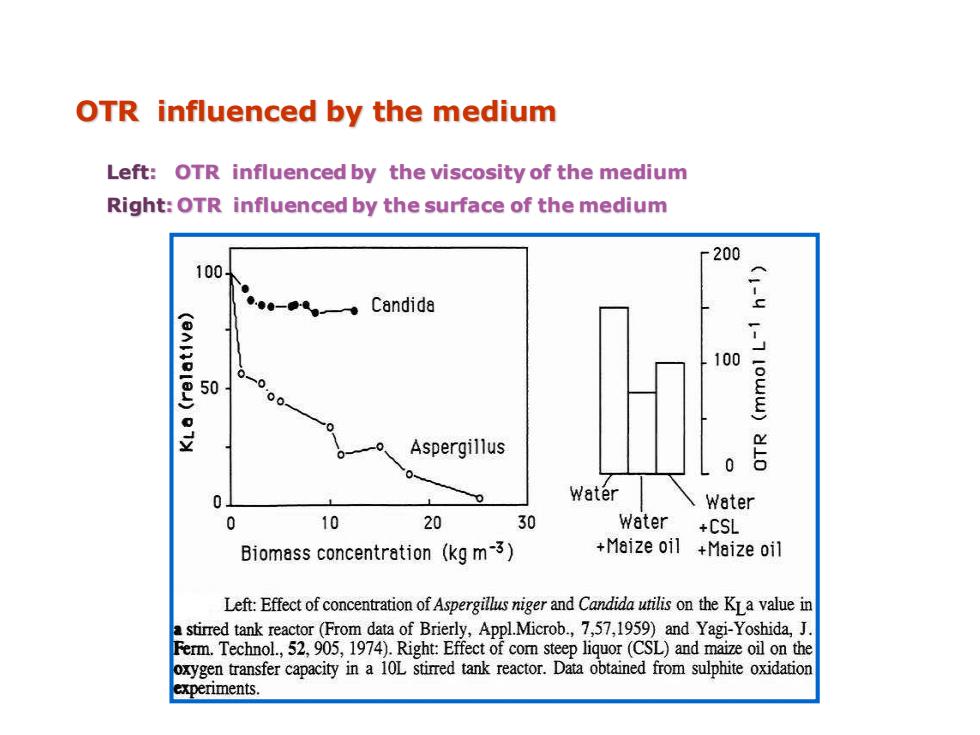
OTR influenced by the medium Left:OTR influenced by the viscosity of the medium Right:OTR influenced by the surface of the medium r200 100. Candide 100 0 00 Aspergillus 0 0 Water Water 0 10 20 30 Water +CSL Biomass concentration (kg m-3) +Maize oil +Maize oil Left:Effect of concentration of Aspergillus niger and Candida utilis on the KLa value in a stirred tank reactor(From data of Brierly,ApplMicrob.,7,57,1959)and Yagi-Yoshida,J. Ferm.Technol.,52,905,1974).Right:Effect of com steep liquor(CSL)and maize oil on the oxygen transfer capacity in a 10L stimed tank reactor.Data obtained from sulphite oxidation experiments
Left: OTR influenced by the viscosity of the medium Right: OTR influenced by the surface of the medium OTR influenced by the medium
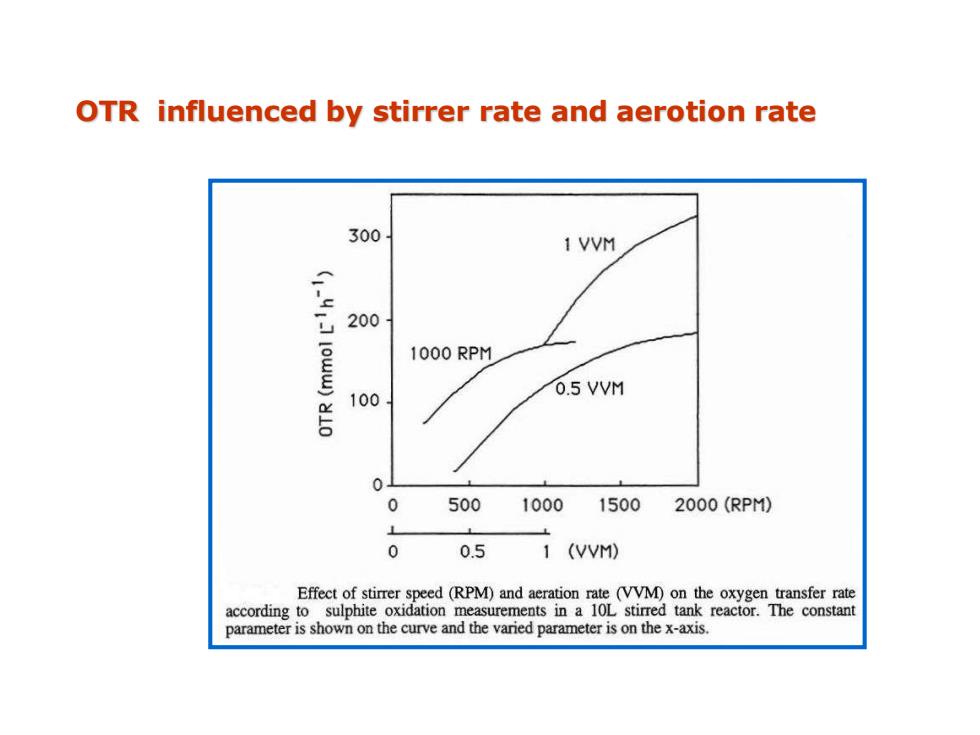
OTR influenced by stirrer rate and aerotion rate 300 1 VVM 200 1000 RPM 100 0.5 VVM 0 0 500 1000 1500 2000(RPM) 0 0.5 1 (VVM) Effect of stirrer speed(RPM)and aeration rate (VVM)on the oxygen transfer rate according to sulphite oxidation measurements in a 10L stirred tank reactor.The constant parameter is shown on the curve and the varied parameter is on the x-axis
OTR influenced by stirrer rate and aerotion rate

OTR influenced by the partial pressure of oxygen(Po2) Poz can be manipulated in two ways: increase of the aeration rate increase the oxygen concentration in the inlet air 200 100 OTR cPlor 0 0123456789 Total gas pressure(bar) Effect of total air pressure on the oxygen transfer rate according to7.OTR is set as 100 at I bar total pressure.The calculation assumes that only oxygen partial pressure and gas bubble volume is influenced by the increased pressure
3 OTR Ptot OTR influenced by the partial pressure of oxygen(PO2) ✓ increase of the aeration rate ✓ increase the oxygen concentration in the inlet air PO2 can be manipulated in two ways :