
Medical Microbiology Laboratory Cover A Digital Manual for Medical Exercisel Exercise2 Microbiology Laboratory. Exercise3 Exercise4 By Dr.Marion Chan,Ph.D. Exercise5 The manual is constructed as a collaborative effort of the Department of Microbiology and Immunology,School of Medicine and School of Podiatric Exercise6 Medicine,Temple University.Colored images of cultures and reactions are provided to allow students to prepare for the exercises as well as review them for Exercise7 exams Clinical Correspondence: case studies Suggestions Dr.Marion Chan,Ph.D.,Assistant Professor,Department of Microbiology and Immunology,Temple University School of Medicine,3400 North Broad Hit Street,Philadelphia,PA,19140. Te:215-707-82620r215-625-5270 marionc@astro.temple.edu Acknowledgement: The authors wish to thank Mr.Ernesto Mu ujorra and Mr.Victor Thompson for their excellent assistance in obtaining some of the microscopic and macroscopic images Drs.Robert Christman and David Grainger for assistance in computer technology Dr.R.Tuan of Thomas Jefferson University and Division of Life Science of Rutgers University have generously allowed us to use their equipment for obtaining the microscopic images. Copyright of Temple University.All rights reserved. Last modified:Sunday September 09,2001. file://CJ/WINDOWS/D sktop/clown/Microb Lab Web 2001/medicalmic ologylaboratory2001.htm [8/31/2002 4:29:46 PM)
Cover Exercise1 Exercise2 Exercise3 Exercise4 Exercise5 Exercise6 Exercise7 Clinical case studies Suggestions Hit Counter A Digital Manual for Medical Microbiology Laboratory. By Dr. Marion Chan, Ph.D. The manual is constructed as a collaborative effort of the Department of Microbiology and Immunology, School of Medicine and School of Podiatric Medicine, Temple University. Colored images of cultures and reactions are provided to allow students to prepare for the exercises as well as review them for exams. Correspondence: Dr. Marion Chan, Ph.D., Assistant Professor, Department of Microbiology and Immunology, Temple University School of Medicine, 3400 North Broad Street, Philadelphia, PA, 19140. Tel: 215-707-8262 or 215-625-5270 marionc@astro.temple.edu Acknowledgement: The authors wish to thank Mr. Ernesto Mujorra and Mr. Victor Thompson for their excellent assistance in obtaining some of the microscopic and macroscopic images. Drs. Robert Christman and David Grainger for assistance in computer technology Dr. R. Tuan of Thomas Jefferson University and Division of Life Science of Rutgers University have generously allowed us to use their equipment for obtaining the microscopic images. Copyright of Temple University. All rights reserved. Last modified: Sunday September 09, 2001. Medical Microbiology Laboratory file:///C|/WINDOWS/Desktop/clown/Microb Lab Web 2001/medicalmicrobiologylaboratory2001.htm [8/31/2002 4:29:46 PM]

Exercise 1 Cover EXERCISE #1 MICROSCOPY,BACTERIAL ISOLATION AND STAINING. Exercisel FIRST SESSION Exercise2 FOCI:Aseptic Technique,Staining,Microscopic Observation of Bacteria Exercise3 and Bacterial Isolation Using Streak Plate Method. Exercise4 A.Aseptic Technique and Isolation of Bacteria colonies Exercise5 Man harbors a wide variety of microorganisms on the skin and in the oral cavity,nasopharynx and intestinal tract.In all these areas of the body,many Exercise6 different kinds of bacteria exist together.Thus,if they are to be studied individually,each species must be isolated and then sub-cultured to obtain a Exercise7 pure culture.This is particularly true in the study of disease where it is Clinical necessary to isolate and identify the specific etiologic agent.Each of the case studies students is to develop lab skills that are collectively referred to as aseptic technique.This concept must be consistently kept in mind when handling Suggestions bacterial cultures because the careless student will contaminate everything including the pure cultures of bacteria provided for the study of general Hit Counter bacteriology.Without pure cultures this study becomes an effort in futility Aseptic technique will be demonstrated by the lab instructor and the studen should become quite familiar with the procedure to aseptically transfer bacteria from pure cultures. In general,pure cultures of isolated bacterial colonies are best obtained by using solid media and streak plates are primarily used for this purpose. y Each student will receive a tryptic soy agar plate and a tube of broth containing a mixture of bac eria.The "fou -way "method will be used to streak the plate.This will be demonstrated by the instructor and is described below. 1.Never remove the top of the plate but lift the lid just high enough to make complete strokes 2.Place a loop of organisms on one edge of the agar(area 1)and then streak back and forth,overlapping the streaks until you have covered about one fourth of the plate. 3.Flame the loop before proceeding to each of the other steps 4.Turn the plate approximately 90 and start streaking from one corner of the streaked area 1 and cover about one fourth of the surface(area 2).Turn again and streak surface area 3 by overlapping area 2,as shown below. Repeat this method and streak the surface of area 4 but do not run the final streaks back into any of the previous areas.You have now spread the file://C/WINDOWS/Desktop/clown/Microb Lab Web 2001/Exercise1/exercise1b.htm(1 of6)[8/3/00 9:54 PM
Cover Exercise1 Exercise2 Exercise3 Exercise4 Exercise5 Exercise6 Exercise7 Clinical case studies Suggestions Hit Counter EXERCISE #1 MICROSCOPY, BACTERIAL ISOLATION AND STAINING. FIRST SESSION FOCI: Aseptic Technique, Staining, Microscopic Observation of Bacteria, and Bacterial Isolation Using Streak Plate Method. A. Aseptic Technique and Isolation of Bacteria colonies Man harbors a wide variety of microorganisms on the skin and in the oral cavity, nasopharynx and intestinal tract. In all these areas of the body, many different kinds of bacteria exist together. Thus, if they are to be studied individually, each species must be isolated and then sub-cultured to obtain a pure culture. This is particularly true in the study of disease where it is necessary to isolate and identify the specific etiologic agent. Each of the students is to develop lab skills that are collectively referred to as aseptic technique. This concept must be consistently kept in mind when handling bacterial cultures because the careless student will contaminate everything, including the pure cultures of bacteria provided for the study of general bacteriology. Without pure cultures this study becomes an effort in futility! Aseptic technique will be demonstrated by the lab instructor and the student should become quite familiar with the procedure to aseptically transfer bacteria from pure cultures. In general, pure cultures of isolated bacterial colonies are best obtained by using solid media and streak plates are primarily used for this purpose. >>>>> Each student will receive a tryptic soy agar plate and a tube of broth containing a mixture of bacteria. The "four-way" method will be used to streak the plate. This will be demonstrated by the instructor and is described below. 1. Never remove the top of the plate but lift the lid just high enough to make complete strokes. 2. Place a loop of organisms on one edge of the agar (area 1) and then streak back and forth, overlapping the streaks until you have covered about one fourth of the plate. 3. Flame the loop before proceeding to each of the other steps 4. Turn the plate approximately 90° and start streaking from one corner of the streaked area 1 and cover about one fourth of the surface (area 2). Turn again and streak surface area 3 by overlapping area 2, as shown below. Repeat this method and streak the surface of area 4 but do not run the final streaks back into any of the previous areas. You have now spread the Exercise 1 file:///C|/WINDOWS/Desktop/clown/Microb Lab Web 2001/Exercise1/exercise1b.htm (1 of 6) [8/31/2002 4:29:54 PM]
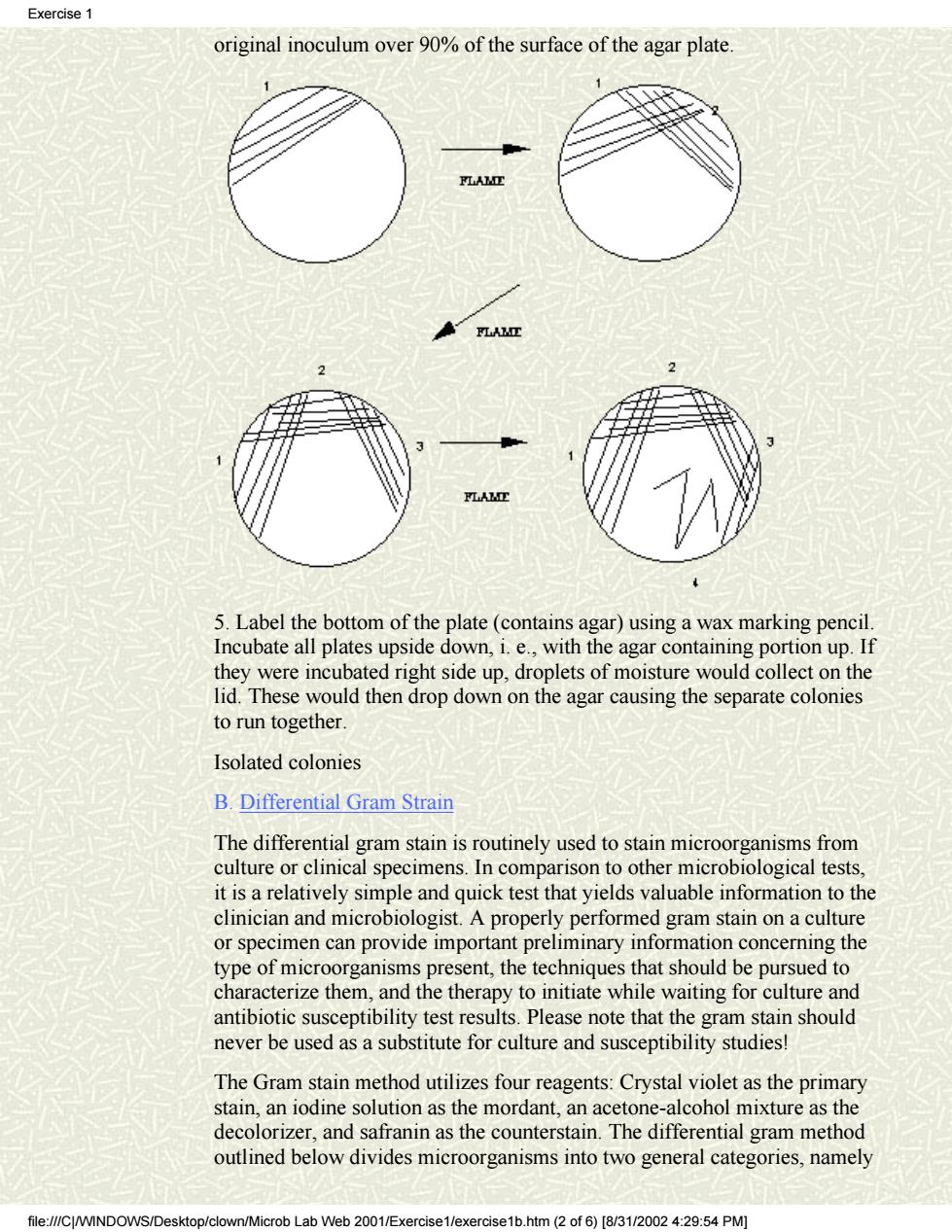
Exercise original inoculum over 90%of the surface of the agar plate 5.Label the bottom of the plate(contains agar)using a wax marking pencil Incubate all plates upside down,i.e.,with the agar containing portion up.If they were incubated right side up,droplets of moisture would collect on the lid.These would then drop down on the agar causing the separate colonies to run together Isolated colonies B.Differential Gram Strain The differential gram stain is routinely used to stain microorganisms from culture or clinical specimens.In comparison to other microbiological tests. it is a relatively simple and quick test that yields valuable information to the clinician and microbiologist.A properly performed gram stain on a culture or specimen can provide important preliminary information concerning the type of microorganisms present,the techniques that should be pursued to characterize them,and the therapy to initiate while waiting for culture and antibiotic susceptibility test results.Please note that the gram stain should never be used as a substitute for culture and susceptibility studies! The Gram stain method utilizes four reagents:Crystal violet as the primary stain,an iodine solution as the mordant,an acetone-alcohol mixture as the decolorizer,and safranin as the counterstain.The differential gram method outlined below divides microorganisms into two general categories,namely rob Lab Web 2001/E× htm2of6)[831/20024:29:54PM
original inoculum over 90% of the surface of the agar plate. 5. Label the bottom of the plate (contains agar) using a wax marking pencil. Incubate all plates upside down, i. e., with the agar containing portion up. If they were incubated right side up, droplets of moisture would collect on the lid. These would then drop down on the agar causing the separate colonies to run together. Isolated colonies B. Differential Gram Strain The differential gram stain is routinely used to stain microorganisms from culture or clinical specimens. In comparison to other microbiological tests, it is a relatively simple and quick test that yields valuable information to the clinician and microbiologist. A properly performed gram stain on a culture or specimen can provide important preliminary information concerning the type of microorganisms present, the techniques that should be pursued to characterize them, and the therapy to initiate while waiting for culture and antibiotic susceptibility test results. Please note that the gram stain should never be used as a substitute for culture and susceptibility studies! The Gram stain method utilizes four reagents: Crystal violet as the primary stain, an iodine solution as the mordant, an acetone-alcohol mixture as the decolorizer, and safranin as the counterstain. The differential gram method outlined below divides microorganisms into two general categories, namely Exercise 1 file:///C|/WINDOWS/Desktop/clown/Microb Lab Web 2001/Exercise1/exercise1b.htm (2 of 6) [8/31/2002 4:29:54 PM]

Exercise 1 gram-positive and gram-negative bacteria.The difference between these bacteria is that gram positives are not decolorized when alcohol or acetone is applied to the smear.They thus retain the primary stain and are colored purple when observed microscopically under oil immersion.Gram-negative bacteria,however,lose the primary stain upon decolorization and appear pink or red because of the safranin counterstain. >>>>> 1.To prepare a smear from a broth culture(liquid suspension of bacterial cells),merely spread a loopful of the culture over a 2 cm square area of the microscope slide.Aseptic technique must be used to accomplish this transfer and remember to ALWAYS FLAME LOOP BEFORE AND AFTER USE!! 2.For colonies on agar plate,place a loop(or two)of water on a glass slide Using aseptic technique,gently touch the bacterial surface growth with an inoculating needle or loop and remove a small part of the colony.Emulsify the bacteria on the loop into the drop of water until it just looks cloudy. Flame the loop to incinerate excess bacteria and allow it to cool.Continue to spread the smear with the loop until you obtain an even distribution of organisms over a 2 cm square area and remember to FLAME YOUR LOOP BEFORE PUTTING IT DOWN ON THE BENCH TOP. 3.Allow the smears to air dry(you may speed the drying by holding the slide high above the flame but do not allow them to become hot). 4.Heat fix the dried bacterial smears by passing the glass slides quickly through the flame two or three times. 5.Stain and observe the microscopic morphology and staining characteristics of each microorganism Gram stain Place the slide on the staining rack and stain the films according to the following method: (1)Crystal violet,1-2 minutes (2)Rinse in tap water and blot dry (3)Gram's iodine,1 minute (4)Rinse in tap water and drain excess water (5)Tilt the slide and add acetone-alcohol,drop by drop,until it flows colorlessly (about 5-10 seconds) (6)Rinse in tap water and drain excess water (7)Safranin,30 seconds (8)Rinse in tap water and blot dry (9)Examine with the oil immersion lens and draw the microscopic morphology microscopic morphology file://CWINDOWS/Desktop/clown/Microb Lab Web 2001/Exercise1/exercise1b.htm (3of 6)[8/3/0024:9:54 PM)
gram-positive and gram-negative bacteria. The difference between these bacteria is that gram positives are not decolorized when alcohol or acetone is applied to the smear. They thus retain the primary stain and are colored purple when observed microscopically under oil immersion. Gram-negative bacteria, however, lose the primary stain upon decolorization and appear pink or red because of the safranin counterstain. >>>>> 1. To prepare a smear from a broth culture (liquid suspension of bacterial cells), merely spread a loopful of the culture over a 2 cm square area of the microscope slide. Aseptic technique must be used to accomplish this transfer and remember to ALWAYS FLAME LOOP BEFORE AND AFTER USE!! 2. For colonies on agar plate, place a loop (or two) of water on a glass slide. Using aseptic technique, gently touch the bacterial surface growth with an inoculating needle or loop and remove a small part of the colony. Emulsify the bacteria on the loop into the drop of water until it just looks cloudy. Flame the loop to incinerate excess bacteria and allow it to cool. Continue to spread the smear with the loop until you obtain an even distribution of organisms over a 2 cm square area and remember to FLAME YOUR LOOP BEFORE PUTTING IT DOWN ON THE BENCH TOP. 3. Allow the smears to air dry (you may speed the drying by holding the slide high above the flame but do not allow them to become hot). 4. Heat fix the dried bacterial smears by passing the glass slides quickly through the flame two or three times. 5. Stain and observe the microscopic morphology and staining characteristics of each microorganism. Gram stain Place the slide on the staining rack and stain the films according to the following method: (1) Crystal violet, 1-2 minutes (2) Rinse in tap water and blot dry (3) Gram's iodine, 1 minute (4) Rinse in tap water and drain excess water (5) Tilt the slide and add acetone-alcohol, drop by drop, until it flows colorlessly (about 5-10 seconds) (6) Rinse in tap water and drain excess water (7) Safranin, 30 seconds (8) Rinse in tap water and blot dry (9) Examine with the oil immersion lens and draw the microscopic morphology microscopic morphology Exercise 1 file:///C|/WINDOWS/Desktop/clown/Microb Lab Web 2001/Exercise1/exercise1b.htm (3 of 6) [8/31/2002 4:29:54 PM]

Exercise C.Brightfield Microscopy 1.Compound microscope Since all the living forms(microbes)with which we will be dealing are invisible to the naked eye,a microscope is an essential tool in any laboratory course in microbiology.The compound microscope employs two lens systems:the ocular or eyepiece and the objective lens.These two lens systems are separated so that the ocular lens can magnify the image formed y the objective lens.An object which is magnified 45 times by the high-dry objective lens(45x)is actually magnified 450 times when combined with the magnification provided by the ocular lens which is usually 10x.A bacterial cell with a linear diameter of 0.001 mm(1 u)can thus be magnified to the point of being visible in the microscope(0.001 mm times 450 equals 0.45 mm). The resolving power of the compound microscope is approximately 0.2u (0.0002 mm).This means that objects which are smaller than 0.2 u be distinguished clearly by the lenses and these will appear as small blurs in the field.Increased magnification will only make the blurs larger.Objects which are less than 0.2 u in diameter thus cannot be resolved by the optical microscope. 2.Use and Care of the Microscope a.Never look through eyepiece when turning adjustment knobs downward You should always begin focusing upward.Otherwise,the objective lens will go through the glass slide,thereby scratching and damaging the expensive lenses of the high-dry and oil-immersion objectives. b.Lenses should be cleaned with a lens cleaning solution and lens paper each day before and after use.Xylene can be used to remove any oil film buildup on the lenses,but it should be used sparingly and must be removed by wiping with lens tissue (xylene is a solvent which can dissolve the cement that holds the lens in the objective). 3.Oil immersion Technique a.Place a drop of immersion oil over the specimen that has been previously fixed onto a glass slide b.Using the course adjustment knob,move the oil-immersion objective (100x)into the oil and gently make contact with the slide(watch from side of scope during this procedure). c.Focusing of specimen is accomplished by turning the fine adjustment knob in a direction which makes the objective travel upward! d.If your microscope is parfocal,you can focus a specimen using the high dry objective,and then place a drop of oil on the specimen,switch to the crob Lab We 2001/Exercise1/e> 1b.htm(4of6)[831/20024:29:54PM
C. Brightfield Microscopy 1. Compound microscope Since all the living forms (microbes) with which we will be dealing are invisible to the naked eye, a microscope is an essential tool in any laboratory course in microbiology. The compound microscope employs two lens systems: the ocular or eyepiece and the objective lens. These two lens systems are separated so that the ocular lens can magnify the image formed by the objective lens. An object which is magnified 45 times by the high-dry objective lens (45x) is actually magnified 450 times when combined with the magnification provided by the ocular lens which is usually 10x. A bacterial cell with a linear diameter of 0.001 mm (1 u) can thus be magnified to the point of being visible in the microscope (0.001 mm times 450 equals 0.45 mm). The resolving power of the compound microscope is approximately 0.2u (0.0002 mm). This means that objects which are smaller than 0.2 u cannot be distinguished clearly by the lenses and these will appear as small blurs in the field. Increased magnification will only make the blurs larger. Objects which are less than 0.2 u in diameter thus cannot be resolved by the optical microscope. 2. Use and Care of the Microscope a. Never look through eyepiece when turning adjustment knobs downward. You should always begin focusing upward. Otherwise, the objective lens will go through the glass slide, thereby scratching and damaging the expensive lenses of the high-dry and oil-immersion objectives. b. Lenses should be cleaned with a lens cleaning solution and lens paper each day before and after use. Xylene can be used to remove any oil film buildup on the lenses, but it should be used sparingly and must be removed by wiping with lens tissue (xylene is a solvent which can dissolve the cement that holds the lens in the objective). 3. Oil immersion Technique a. Place a drop of immersion oil over the specimen that has been previously fixed onto a glass slide. b. Using the course adjustment knob, move the oil-immersion objective (100x) into the oil and gently make contact with the slide (watch from side of scope during this procedure). c. Focusing of specimen is accomplished by turning the fine adjustment knob in a direction which makes the objective travel upward! d. If your microscope is parfocal, you can focus a specimen using the high dry objective, and then place a drop of oil on the specimen, switch to the Exercise 1 file:///C|/WINDOWS/Desktop/clown/Microb Lab Web 2001/Exercise1/exercise1b.htm (4 of 6) [8/31/2002 4:29:54 PM]

Exercise 1 oil-immersion objective,and use the fine adjustment to bring specimen into proper focus. e.To remove specimen,turn course adjustment knob in the direction tha makes the objective travel away from the slide.Remove microscope slide eaeswhestscheacpostaaec 10X 40X 60X Oil Immersion 100X SECOND SESSION FOCI:Gram Stain Technique,Characterization of Isolated Colonies A.Colony Morphology of Isolated Colonies and Pure Cultures >> 1.Check the TSA medium plates that were inoculated via the streak plate method for the presence of separate distinct colonies.Describe the cultural characteristics of these isolated colonies as to their size and shape. 2.Pure cultures of individual microorganisms are obtained by picking up a separated colony with a sterile inoculating needle or loop and streaking another plate of medium with this material.After 24 hours of incubation one should have an entire plate of one specific microorganism,and such pure cultures are then used to perform various tests and inoculations so that the bacterium can be identified according to its enzymaticaction on various substrates. 3.Prior to determining the metabolic activity of unknown bacteria, microorganisms are first identified based upon their morphology,namely their staining reaction,size,shape,pigment production,motility,and presence of capsules or spores.Gram stain several of the isolated colonies from the M-H streak plate and attempt to identify them morphologically 4.Pure cultures of Staphylococcus epidermidis and Pseudomonas geruginosa on M-H media will be available for student examination. Describe the cultural colony characteristics of these microorganisms and look for pigment production.Gram stain these bacteria and identify their microscopic morphology. Staphylococcus epidermidis Pseudomonas aeruginosa B.Differential gram stain >>> Prepare a smear of Escherichia coli on one end of the slide and a smear of file:///Desktop/clown/Microb Lab Web 2001/Exercise1/exercise1b.htm(5 of6)[8/3/00 :9:54 PM]
oil-immersion objective, and use the fine adjustment to bring specimen into proper focus. e. To remove specimen, turn course adjustment knob in the direction that makes the objective travel away from the slide. Remove microscope slide and always clean lens with lens tissue before proceeding to examine the next specimen. 10X 40X 60X Oil Immersion 100X SECOND SESSION FOCI: Gram Stain Technique, Characterization of Isolated Colonies A. Colony Morphology of Isolated Colonies and Pure Cultures >>>>> 1. Check the TSA medium plates that were inoculated via the streak plate method for the presence of separate distinct colonies. Describe the cultural characteristics of these isolated colonies as to their size and shape. 2. Pure cultures of individual microorganisms are obtained by picking up a separated colony with a sterile inoculating needle or loop and streaking another plate of medium with this material. After 24 hours of incubation, one should have an entire plate of one specific microorganism, and such pure cultures are then used to perform various tests and inoculations so that the bacterium can be identified according to its enzymatic action on various substrates. 3. Prior to determining the metabolic activity of unknown bacteria, microorganisms are first identified based upon their morphology, namely their staining reaction, size, shape, pigment production, motility, and presence of capsules or spores. Gram stain several of the isolated colonies from the M-H streak plate and attempt to identify them morphologically. 4. Pure cultures of Staphylococcus epidermidis and Pseudomonas aeruginosa on M-H media will be available for student examination. Describe the cultural colony characteristics of these microorganisms and look for pigment production. Gram stain these bacteria and identify their microscopic morphology. Staphylococcus epidermidis Pseudomonas aeruginosa B. Differential gram stain >>>>> Prepare a smear of Escherichia coli on one end of the slide and a smear of Exercise 1 file:///C|/WINDOWS/Desktop/clown/Microb Lab Web 2001/Exercise1/exercise1b.htm (5 of 6) [8/31/2002 4:29:54 PM]

Exercise1 Staphylococcus epidermidis on the other end of the slide.Preparing a smear from an agar culture(surface bacterial growth on solid medium)is a little more difficult and takes practice to obtain a good concentration(see SESSION 1:B.2). Gram stain and examine under the microscope as in session one IMAGES: 10X mag 40X mag 60X mag Oil immersion Gram stain of mixed culture S.epidermidis on M-H P.aureginosa on M-H file:///C/WINDOWS/Desktop/clown/Microb Lab Web 2001/Exercise1/exercise1b.htm(6 of 6)[8/31/2002 4:29:54 PM]
Staphylococcus epidermidis on the other end of the slide. Preparing a smear from an agar culture (surface bacterial growth on solid medium) is a little more difficult and takes practice to obtain a good concentration (see SESSION 1: B.2). Gram stain and examine under the microscope as in session one. IMAGES: 10X mag 40X mag 60X mag Oil immersion Gram stain of mixed culture S. epidermidis on M-H P. aureginosa on M-H Exercise 1 file:///C|/WINDOWS/Desktop/clown/Microb Lab Web 2001/Exercise1/exercise1b.htm (6 of 6) [8/31/2002 4:29:54 PM]
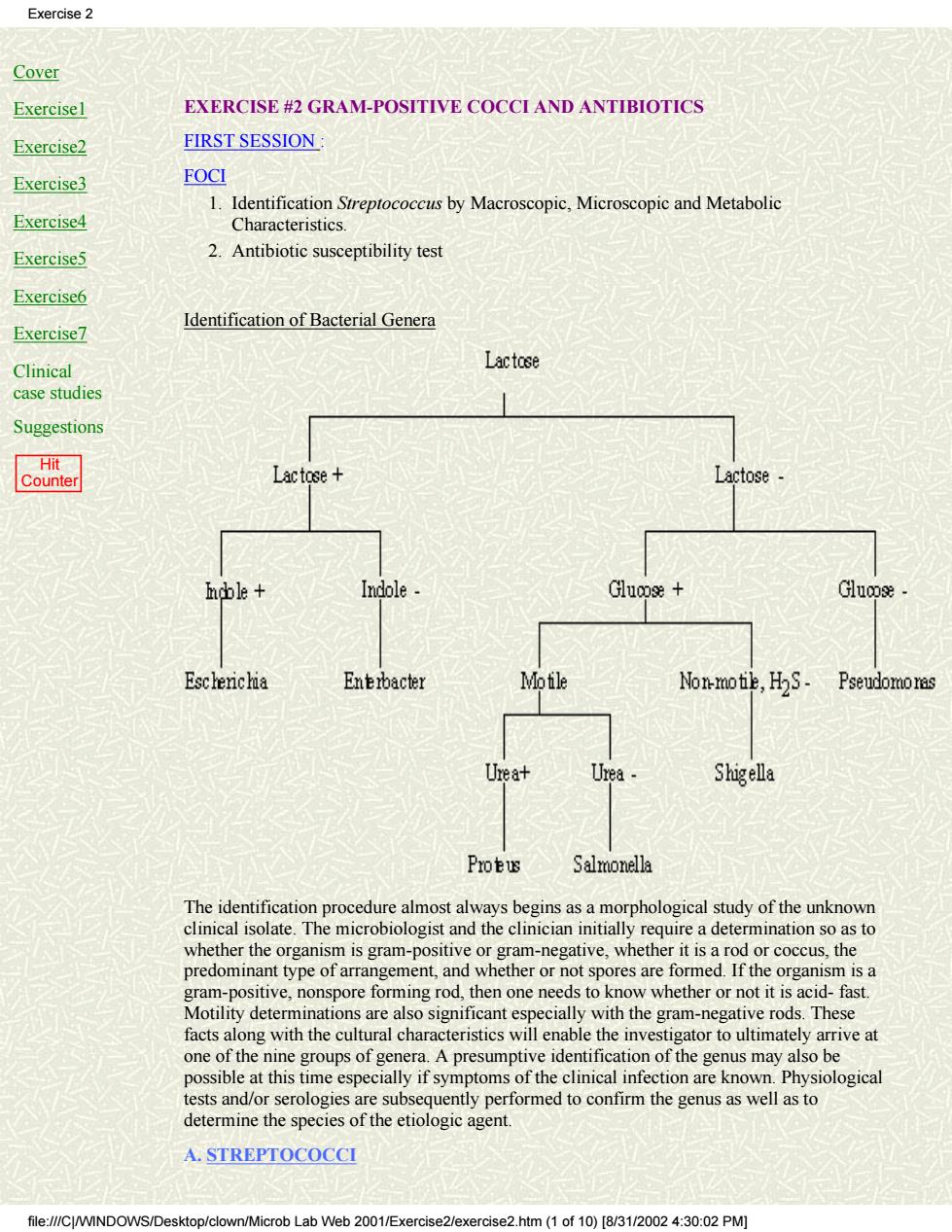
Exercise 2 Cover Exercise1 EXERCISE #2 GRAM-POSITIVE COCCI AND ANTIBIOTICS Exercise2 FIRST SESSION Exercise3 FOCI 1.Identification Streptococcus by Macroscopic,Microscopic and Metabolic Exercise4 Characteristics Exercise5 2.Antibiotic susceptibility test Exercise6 Exercise7 Identification of Bacterial Genera Clinical Lactose case studies Suggestions Lactose+ Lactose Indole Glucose+ Escherichia Enebacter Mofile Non-motie,HS- Pseudomoras Urea+ Urea Shigella Protus Salmonella alm ost always bes gins as a m rphological study of the unknown sand the clincin nta requrea detot organism is a gram-positive,nonspore forming rod,then one needs to know whether or not it is acid-fast. Motility determinations are also significant especially with the gram-negative rods.These facts along with the cultural characteristics will enable the investigator to ultimately arrive at one of the nine groups of genera.A presumptive identification of the genus may also be possible at this time especially if symptoms of the clinical infection are known.Physiological tests and/or serologies are subsequently performed to confirm the genus as well as to determine the species of the etiologic agent. A.STREPTOCOCCI file://CINDOWS/Desktop/clown/Microb Lab Web 2001/Exercise2/exercise2.htm(1 of10)[/3/0:0:02 PM]
Cover Exercise1 Exercise2 Exercise3 Exercise4 Exercise5 Exercise6 Exercise7 Clinical case studies Suggestions Hit Counter EXERCISE #2 GRAM-POSITIVE COCCI AND ANTIBIOTICS FIRST SESSION : FOCI Identification Streptococcus by Macroscopic, Microscopic and Metabolic Characteristics. 1. 2. Antibiotic susceptibility test Identification of Bacterial Genera The identification procedure almost always begins as a morphological study of the unknown clinical isolate. The microbiologist and the clinician initially require a determination so as to whether the organism is gram-positive or gram-negative, whether it is a rod or coccus, the predominant type of arrangement, and whether or not spores are formed. If the organism is a gram-positive, nonspore forming rod, then one needs to know whether or not it is acid- fast. Motility determinations are also significant especially with the gram-negative rods. These facts along with the cultural characteristics will enable the investigator to ultimately arrive at one of the nine groups of genera. A presumptive identification of the genus may also be possible at this time especially if symptoms of the clinical infection are known. Physiological tests and/or serologies are subsequently performed to confirm the genus as well as to determine the species of the etiologic agent. A. STREPTOCOCCI Exercise 2 file:///C|/WINDOWS/Desktop/clown/Microb Lab Web 2001/Exercise2/exercise2.htm (1 of 10) [8/31/2002 4:30:02 PM]

Exercise 2 Sreptococci are gram-positive cocci which typically occur in chains,clusters,and pairs. The that fo in nt hac the mout orm the dom ial flora of gastrointestinal tract Streptococcus pyogenes (Lancefield's GroupA,beta-hemolytic streptococci)is the most reknown pathogen in this genus.It causes infections such as acute pharyngitis(strep sore throat),impetigo,post-operative wound infections,cellulitis,erysipelas,post-streptococcal glomerulonephritis,and acute rheumatic fever.Asymptomatic colonization of S.pyogenes in the pharynx and on skin surfaces as well as anal carriage is not uncommon and may serve as a source of infection. Streptococcus agalactiae(Lancefield's Group B,beta-hemolytic streptococci)can be vered from the g nital and intestinal t arently healthy adults and children.It is primarily i ated i natal infectio s pn ia and/or he it ha Enterococcus faecalis(Lancefield's Group D,nonhemolytic streptococci)is normally found in the intestinal tract along with other enterococci(E.faecium and E.durans).It causes infections such as subacute bacterial endocarditis(20%),urinary tract infections(10%), wound infections,and some other opportunistic infections.The resistance of enterococcal species to penicillin is an important fact to remember in treating patients infected with these streptococci. eumoniae can be isolated from the pharynx of 30-70%of apparently healthy rimarily causes a bacterial r monia ially in the debilitated or compr atients with conjunctivitis meningitis,otitis,pericarditis,empyemaendocarditis,and septicemia Viridans streptococci includes about 10 species(S.mutans.S.sanguis.S.salivarius)which are normally found in the mouth and pharynx.These streptococci are usually alpha or non-hemolytic on blood agar and cause about 50-70%of all subacute bacterial endocarditis They have also been implicated in causing dental caries,bacteremia,and sepsis. Blood agar plate cultures of each of the following microorganisms will be available for student examination a Streptococcus(Group A Streptococci) b.Streptococcus agalactiae (Group B Streptococci) c.Streptococcus pneumoniae(Pneumococci) d.Enterococcus faecalis(Group D Streptococci) >>> Each student should use one of these cultures to perform the following tests. 1.Describe the cultural characteristics of the various streptococci on blood agar medium Note the type.size.and color of the colonies and describe the various types of hemolysis observed with these microorganisms. Streptococcus pyogenes Streptococcus agalactiae Streptococcus pneumoniae Enterococcus faecalis file://CWIND n/Microb Lab Web 2001/Exercise2/exercise2.htm(2 of 10)[8/31/2002 4:30:02 PM]
Streptococci are gram-positive cocci which typically occur in chains, clusters, and pairs. There are many members of the genus Streptococcus that form the dominant bacterial flora of the mouth and the pharynx. Some streptococcal species are normally found in the gastrointestinal tract. Streptococcus pyogenes (Lancefield's GroupA, beta-hemolytic streptococci) is the most reknown pathogen in this genus. It causes infections such as acute pharyngitis (strep sore throat), impetigo, post-operative wound infections, cellulitis, erysipelas, post-streptococcal glomerulonephritis, and acute rheumatic fever. Asymptomatic colonization of S. pyogenes in the pharynx and on skin surfaces as well as anal carriage is not uncommon and may serve as a source of infection. Streptococcus agalactiae (Lancefield's Group B, beta-hemolytic streptococci) can be recovered from the genital and intestinal tract of apparently healthy adults and children. It is primarily implicated in causing neonatal infections such as pneumonia, septicemia and/or meningitis. Nevertheless, it has been isolated from patients with urinary tract infections, wound infections and otitis media. Enterococcus faecalis (Lancefield's Group D, nonhemolytic streptococci) is normally found in the intestinal tract along with other enterococci (E. faecium and E. durans). It causes infections such as subacute bacterial endocarditis (20%), urinary tract infections (10%), wound infections, and some other opportunistic infections. The resistance of enterococcal species to penicillin is an important fact to remember in treating patients infected with these streptococci. Streptococcus pneumoniae can be isolated from the pharynx of 30-70% of apparently healthy individuals. It primarily causes a bacterial pneumonia especially in the debilitated or compromised patient. This diplococcus has also been isolated in patients with conjunctivitis, meningitis, otitis, pericarditis, empyema, endocarditis, and septicemia. Viridans streptococci includes about 10 species (S. mutans, S. sanguis, S. salivarius) which are normally found in the mouth and pharynx. These streptococci are usually alpha or non-hemolytic on blood agar and cause about 50-70% of all subacute bacterial endocarditis. They have also been implicated in causing dental caries, bacteremia, and sepsis. Blood agar plate cultures of each of the following microorganisms will be available for student examination: a. Streptococcus (Group A Streptococci) b. Streptococcus agalactiae (Group B Streptococci) c. Streptococcus pneumoniae (Pneumococci) d. Enterococcus faecalis (Group D Streptococci) >>>>> Each student should use one of these cultures to perform the following tests. 1. Describe the cultural characteristics of the various streptococci on blood agar medium. Note the type, size, and color of the colonies and describe the various types of hemolysis observed with these microorganisms. Streptococcus pyogenes Streptococcus agalactiae Streptococcus pneumoniae Enterococcus faecalis Exercise 2 file:///C|/WINDOWS/Desktop/clown/Microb Lab Web 2001/Exercise2/exercise2.htm (2 of 10) [8/31/2002 4:30:02 PM]

Exercise 2 1.Examine bacteria microscopically by preparing a gram stain. Streptococcus pyogenes Streptococcus agalactiae Streptococcus pneumoniae Enterococcus faecalis 2.Catalase Test--Perform the slide test by emulsifying some visible growth material in a drop of hydrogen peroxide and note that all streptococci are catalase-negative(no rapid ebullition). 3.Rapid Enterococcus Identification--The bile esculin(BE)test is the most accurate way for identifying Group D streptococci because they are all BE-positive and will cause a blackening of the agar medium after overnight incubation.All other streptococci are negative for this reaction.An enterococcus/Group A screen will be available for the rapid identification of Enterococcus faecalis from other streptococci.This specific medium contains esculin and also PYR(pyroglutamylbeta-naphthylamide)which will differentiate enterococci from other Group D streptococci. a)Using a sterile lo obtain a visible,he avy ino mn an stab into the medium in an area near one side next to the glass.Incubate the tube at 35C for 30 minutes to 4 hours b)The esculin hydrolysis usually occurs in 30 minutes with a heavy inoculum and is indicated by development of a dark brown color.The PYR reaction is detected by adding 2 drops of the PYR reagent and observing a cherry red color within 2 minutes.No color change,light orange or light pink-orange is considered negative. Positive and Negative Reactions of BE-PYR strep om other het tc streptoc cci by eaction The usually requires the inoculation of bile esculin agar and tube of broth containing6.5%NaCl d)After overnight incubation,the black discoloration of bile esculin identifies Group D streptococci(E.faecalis,S.bovis,etc.)and growth in the presence of 6.5%NaCl (salt tolerance test)differentiates enterococci which are salt tolerant from other Group D streptococci(S.bovis)which will not grow in this broth. Group A Groups GrOUD D GrOuD D B.CF.G Enterococcus Non-Enterococcus Esculin 十 十 PYR x 、 5.Rapid Streptococeal Grouping Most streptococcal species possess group specific antigens which are usually carbohydrate structural components of the cell wall.These antigens can be extracted and identified by homologous antisera,and the bacterium can be assigned to one of the various Lancefield groups.The principal use of the test is to rapidly identify strepto reported with one hour extraction from r wound swabs). Extraction and Test Procedure: a.To a small glass tube,2 drops of reagent I and 2 drops of reagent 2 are added to generate file://CJ/WINDOWS/Desktop/clown/Microb Lab Web 2001/Exercise2/exercise2.htm (3 of 10)[8/31/0024:30:02 PM]
1. Examine bacteria microscopically by preparing a gram stain. Streptococcus pyogenes Streptococcus agalactiae Streptococcus pneumoniae Enterococcus faecalis 2. Catalase Test -- Perform the slide test by emulsifying some visible growth material in a drop of hydrogen peroxide and note that all streptococci are catalase-negative (no rapid ebullition). 3. Rapid Enterococcus Identification -- The bile esculin (BE) test is the most accurate way for identifying Group D streptococci because they are all BE-positive and will cause a blackening of the agar medium after overnight incubation. All other streptococci are negative for this reaction. An enterococcus/Group A screen will be available for the rapid identification of Enterococcus faecalis from other streptococci. This specific medium contains esculin and also PYR (pyroglutamylbeta- naphthylamide) which will differentiate enterococci from other Group D streptococci. a) Using a sterile loop, obtain a visible, heavy inoculum and stab into the medium in an area near one side next to the glass. Incubate the tube at 35°C for 30 minutes to 4 hours. b) The esculin hydrolysis usually occurs in 30 minutes with a heavy inoculum and is indicated by development of a dark brown color. The PYR reaction is detected by adding 2 drops of the PYR reagent and observing a cherry red color within 2 minutes. No color change, light orange or light pink-orange is considered negative. Positive and Negative Reactions of BE-PYR c) From the foregoing table, you should realize that the screen test can also differentiate Group A streptococci from other beta-hemolytic streptococci by the PYR reaction. The identification of Enterococcus faecalis usually requires the inoculation of bile esculin agar and tube of broth containing 6.5% NaCl. d) After overnight incubation, the black discoloration of bile esculin identifies Group D streptococci (E. faecalis, S. bovis, etc.) and growth in the presence of 6.5% NaCl (salt tolerance test) differentiates enterococci which are salt tolerant from other Group D streptococci (S. bovis) which will not grow in this broth. Group A Groups B,C,F,G Group D Enterococcus Group D Non-Enterococcus Esculin - - + + PYR + - - - 5. Rapid Streptococcal Grouping Most streptococcal species possess group specific antigens which are usually carbohydrate structural components of the cell wall. These antigens can be extracted and identified by homologous antisera, and the bacterium can be assigned to one of the various Lancefield groups. The principal use of the test is to rapidly identify streptococci growing on agar plates (but satisfactory results have been reported with one hour extraction from blood cultures and from throat, skin, or wound swabs). Extraction and Test Procedure: a. To a small glass tube, 2 drops of reagent 1 and 2 drops of reagent 2 are added to generate Exercise 2 file:///C|/WINDOWS/Desktop/clown/Microb Lab Web 2001/Exercise2/exercise2.htm (3 of 10) [8/31/2002 4:30:02 PM]