正在加载图片...
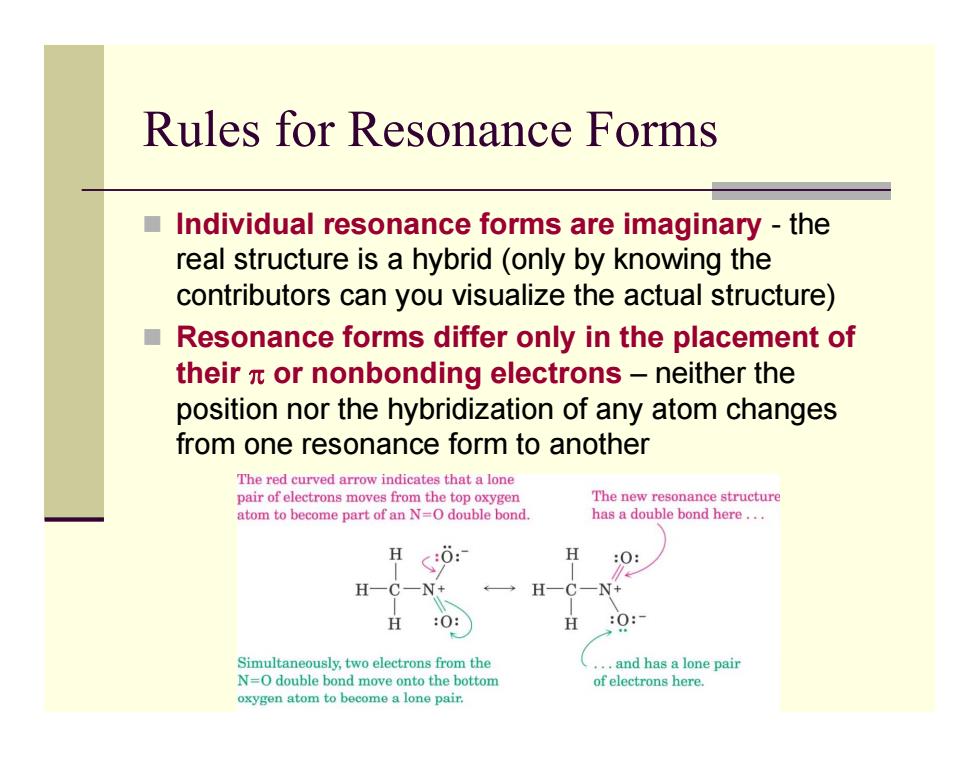
Rules for Resonance Forms Individual resonance forms are imaginary-the real structure is a hybrid (only by knowing the contributors can you visualize the actual structure) ■ Resonance forms differ only in the placement of their x or nonbonding electrons-neither the position nor the hybridization of any atom changes from one resonance form to another The red curved arrow indicates that a lone pair of electrons moves from the top oxygen The new resonance structure atom to become part of an N=O double bond. has a double bond here... H :0: H N+ H 父 O: H 0: Simultaneously,two electrons from the ..and has a lone pair N=O double bond move onto the bottom of electrons here. oxygen atom to become a lone pair. Rules for Resonance Forms Individual resonance forms are imaginary - the real structure is a hybrid (only by knowing the contributors can you visualize the actual structure) Resonance forms differ only in the placement of their π or nonbonding electrons – neither the position nor the hybridization of any atom changes from one resonance form to another