正在加载图片...
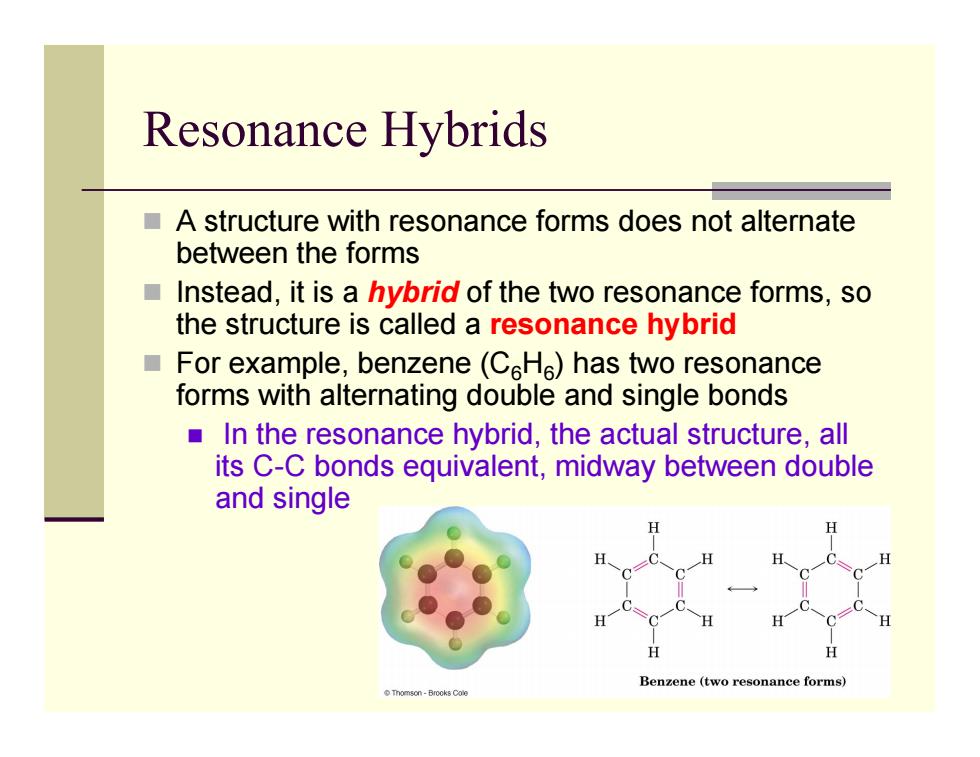
Resonance Hybrids A structure with resonance forms does not alternate between the forms Instead,it is a hybrid of the two resonance forms,so the structure is called a resonance hybrid For example,benzene(CHe)has two resonance forms with alternating double and single bonds In the resonance hybrid,the actual structure,all its C-C bonds equivalent,midway between double and single Benzene (twe nance forms Tomson-Brooks CaleResonance Hybrids A structure with resonance forms does not alternate between the forms Instead, it is a hybrid of the two resonance forms, so the structure is called a resonance hybrid For example, benzene (C 6 H 6) has two resonance forms with alternating double and single bonds In the resonance hybrid, the actual structure, all its C-C bonds equivalent, midway between double and single