正在加载图片...
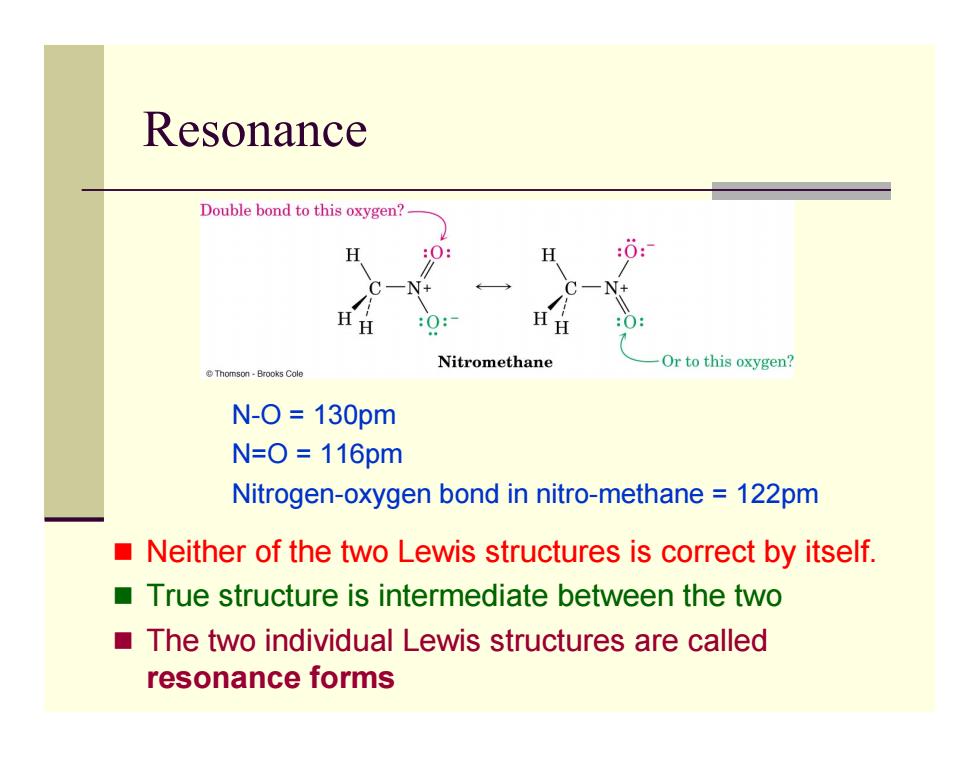
Resonance Double bond to this oxygen? H :0: Nitromethane Or to this oxygen? Thomson-Brooks Cole N-O=130pm N=0=116pm Nitrogen-oxygen bond in nitro-methane 122pm Neither of the two Lewis structures is correct by itself. True structure is intermediate between the two The two individual Lewis structures are called resonance forms Resonance Neither of the two Lewis structures is correct by itself. True structure is intermediate between the two The two individual Lewis structures are called resonance forms N-O = 130pm N=O = 116pm Nitrogen-oxygen bond in nitro-methane = 122pm