正在加载图片...
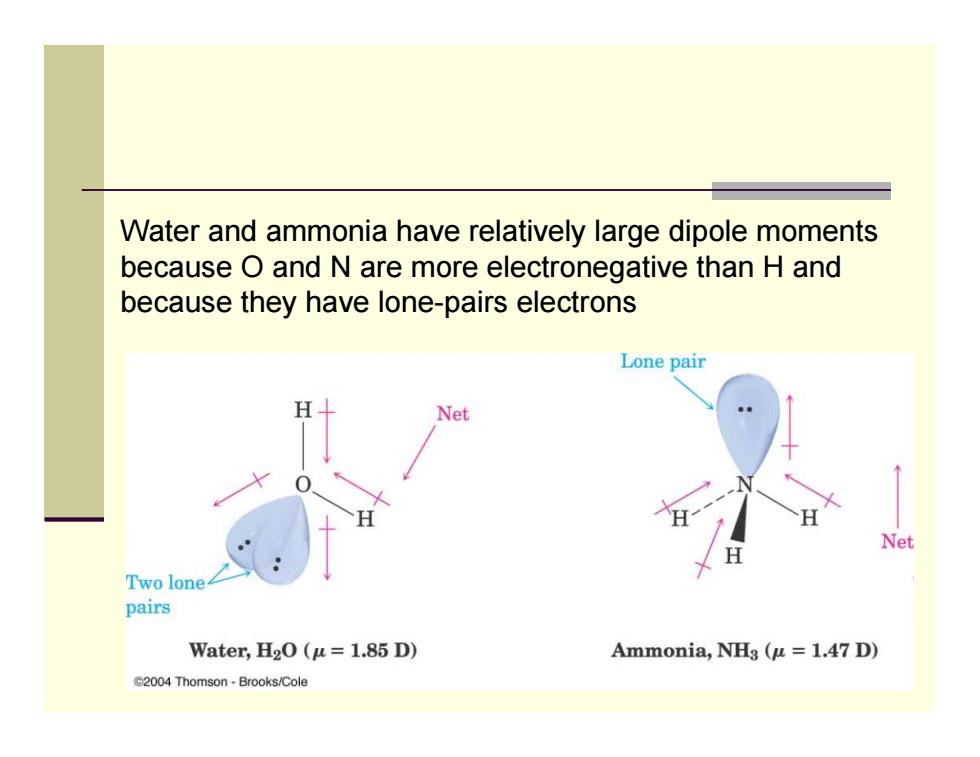
Water and ammonia have relatively large dipole moments because O and N are more electronegative than H and because they have lone-pairs electrons Lone pair Net Net Two lone pairs Water,H2O(u=1.85 D) Ammonia,NHg (u =1.47 D) G2004 Thomson-Brooks/ColeWater and ammonia have relatively large dipole moments because O and N are more electronegative than H and because they have lone-pairs electrons