正在加载图片...
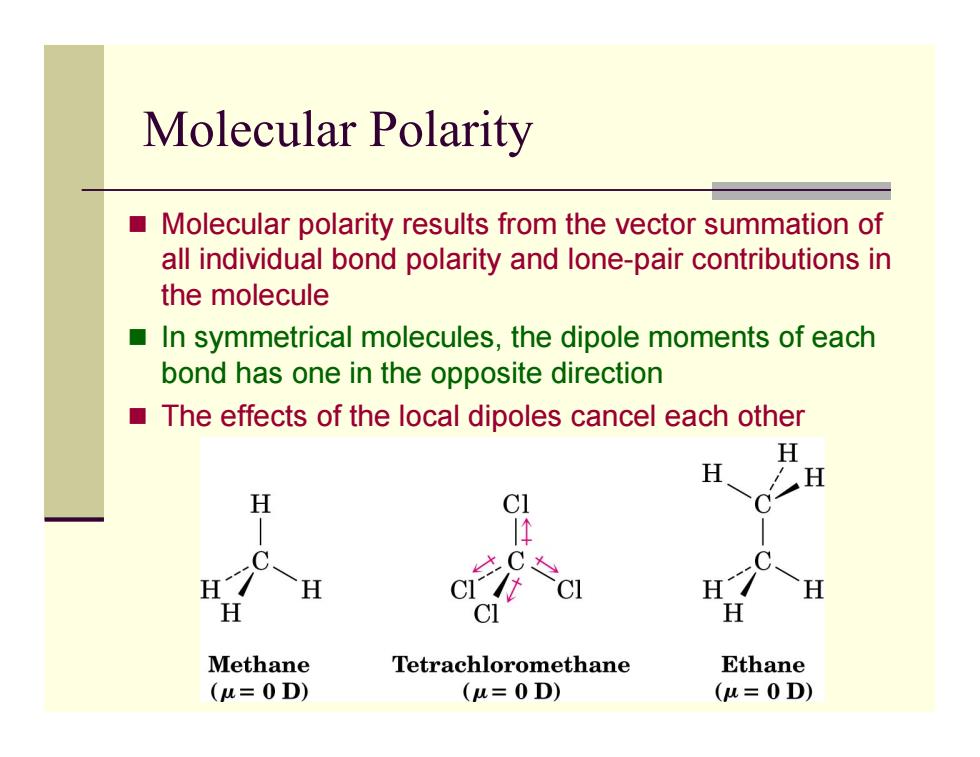
Molecular Polarity Molecular polarity results from the vector summation of all individual bond polarity and lone-pair contributions in the molecule ■ In symmetrical molecules,the dipole moments of each bond has one in the opposite direction The effects of the local dipoles cancel each other H H H H C1 必C& H H H H CI H Methane Tetrachloromethane Ethane (u=0D) (u=0D) (u=0D) Molecular Polarity Molecular polarity results from the vector summation of all individual bond polarity and lone-pair contributions in the molecule In symmetrical molecules, the dipole moments of each bond has one in the opposite direction The effects of the local dipoles cancel each other