正在加载图片...
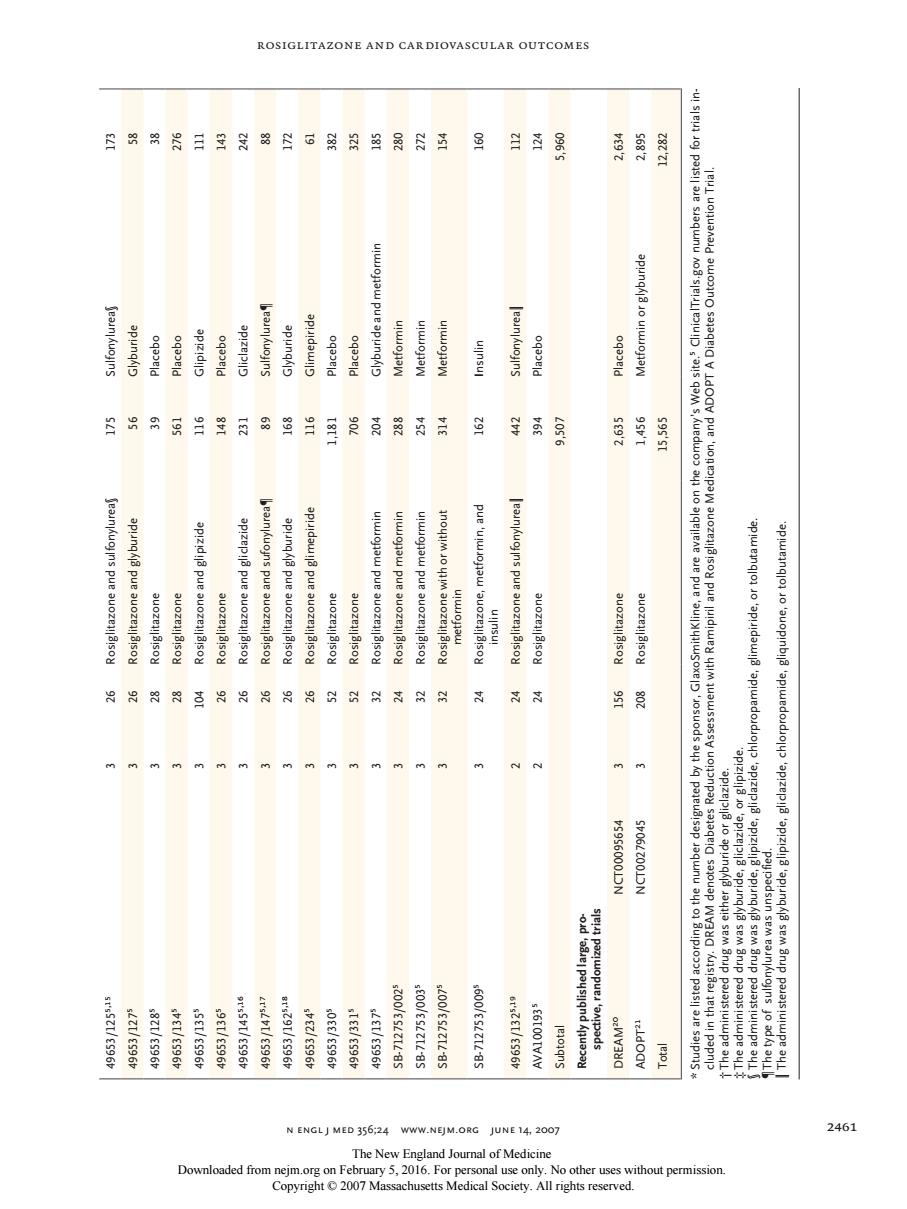
ROSIGLITAZONE AND CARDIOVASCULAR OUTCOMES 图网网闲目生常赠型可的8超用尉五多目百景 及剑 a能服:用 投品份质吕生有8超吕耳发月用日月当羊香 月堂屋 ap/z N ENGLJ MED 356;24 WWW.NEJM.ORG JUNE 14,2007 246 The New England Joumal of Medicine Rosiglitazone and Cardiovascular Outcomes n engl j med 356;24 www.nejm.org june 14 , 2007 2461 49653/1255,15 3 26 Rosiglitazone and sulfonylurea§ 175 Sulfonylurea§ 173 49653/1275 3 26 Rosiglitazone and glyburide 56 Glyburide 58 49653/1285 3 28 Rosiglitazone 39 Placebo 38 49653/1345 3 28 Rosiglitazone 561 Placebo 276 49653/1355 3 104 Rosiglitazone and glipizide 116 Glipizide 111 49653/1365 3 26 Rosiglitazone 148 Placebo 143 49653/1455,16 3 26 Rosiglitazone and gliclazide 231 Gliclazide 242 49653/1475,17 3 26 Rosiglitazone and sufonylurea¶ 89 Sulfonylurea¶ 88 49653/1625,18 3 26 Rosiglitazone and glyburide 168 Glyburide 172 49653/2345 3 26 Rosiglitazone and glimepiride 116 Glimepiride 61 49653/3305 3 52 Rosiglitazone 1,181 Placebo 382 49653/3315 3 52 Rosiglitazone 706 Placebo 325 49653/1375 3 32 Rosiglitazone and metformin 204 Glyburide and metformin 185 SB-712753/0025 3 24 Rosiglitazone and metformin 288 Metformin 280 SB-712753/0035 3 32 Rosiglitazone and metformin 254 Metformin 272 SB-712753/0075 3 32 Rosiglitazone with or without metformin 314 Metformin 154 SB-712753/0095 3 24 Rosiglitazone, metformin, and insulin 162 Insulin 160 49653/1325,19 2 24 Rosiglitazone and sulfonylurea‖ 442 Sulfonylurea‖ 112 AVA1001935 2 24 Rosiglitazone 394 Placebo 124 Subtotal 9,507 5,960 Recently published large, prospective, randomized trials DREAM20 NCT00095654 3 156 Rosiglitazone 2,635 Placebo 2,634 ADOPT21 NCT00279045 3 208 Rosiglitazone 1,456 Metformin or glyburide 2,895 Total 15,565 12,282 * Studies are listed according to the number designated by the sponsor, GlaxoSmithKline, and are available on the company’s Web site.5 ClinicalTrials.gov numbers are listed for trials included in that registry. DREAM denotes Diabetes Reduction Assessment with Ramipiril and Rosiglitazone Medication, and ADOPT A Diabetes Outcome Prevention Trial. † The administered drug was either glyburide or gliclazide. ‡ The administered drug was glyburide, gliclazide, or glipizide. § The administered drug was glyburide, glipizide, gliclazide, chlorpropamide, glimepiride, or tolbutamide. ¶The type of sulfonylurea was unspecified. ‖ The administered drug was glyburide, glipizide, gliclazide, chlorpropamide, gliquidone, or tolbutamide. The New England Journal of Medicine Downloaded from nejm.org on February 5, 2016. For personal use only. No other uses without permission. Copyright © 2007 Massachusetts Medical Society. All rights reserved