正在加载图片...
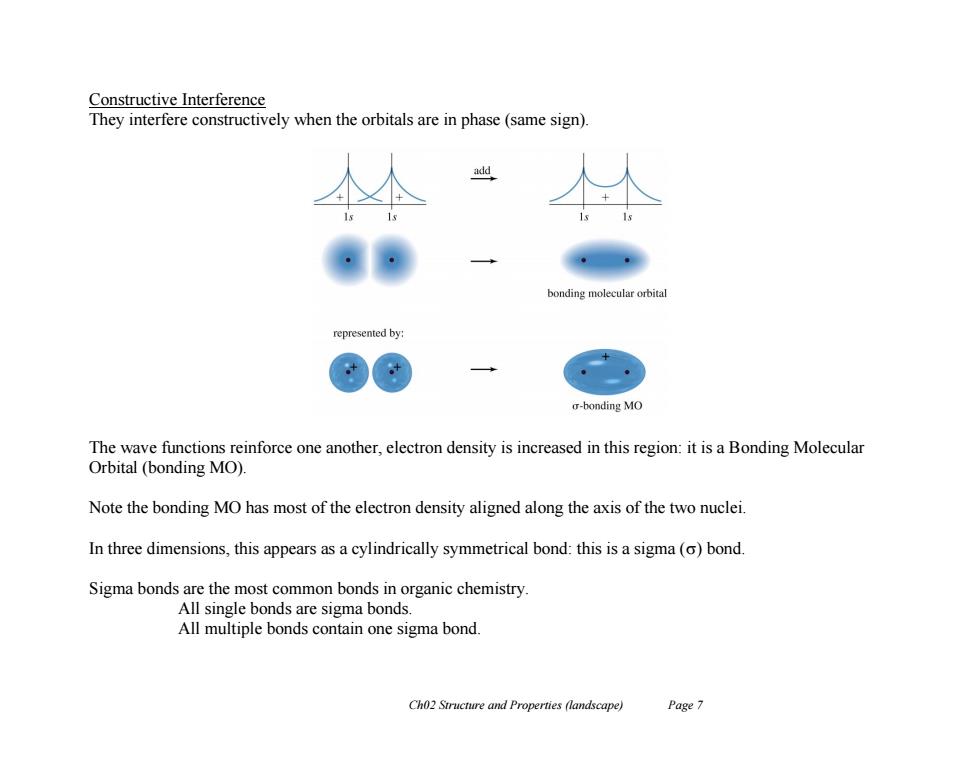
Constructive Interference They interfere constructively when the orbitals are in phase(same sign). add bonding molecular orbital represented by: o-bonding MO The wave functions reinforce one another,electron density is increased in this region:it is a Bonding Molecular Orbital (bonding MO) Note the bonding MO has most of the electron density aligned along the axis of the two nuclei. In three dimensions,this appears as a cylindrically symmetrical bond:this is a sigma(o)bond. Sigma bonds are the most common bonds in organic chemistry. All single bonds are sigma bonds. All multiple bonds contain one sigma bond. Ch02 Structure and Properties (landscape) Page 7Ch02 Structure and Properties (landscape) Page 7 Constructive Interference They interfere constructively when the orbitals are in phase (same sign). The wave functions reinforce one another, electron density is increased in this region: it is a Bonding Molecular Orbital (bonding MO). Note the bonding MO has most of the electron density aligned along the axis of the two nuclei. In three dimensions, this appears as a cylindrically symmetrical bond: this is a sigma () bond. Sigma bonds are the most common bonds in organic chemistry. All single bonds are sigma bonds. All multiple bonds contain one sigma bond