正在加载图片...
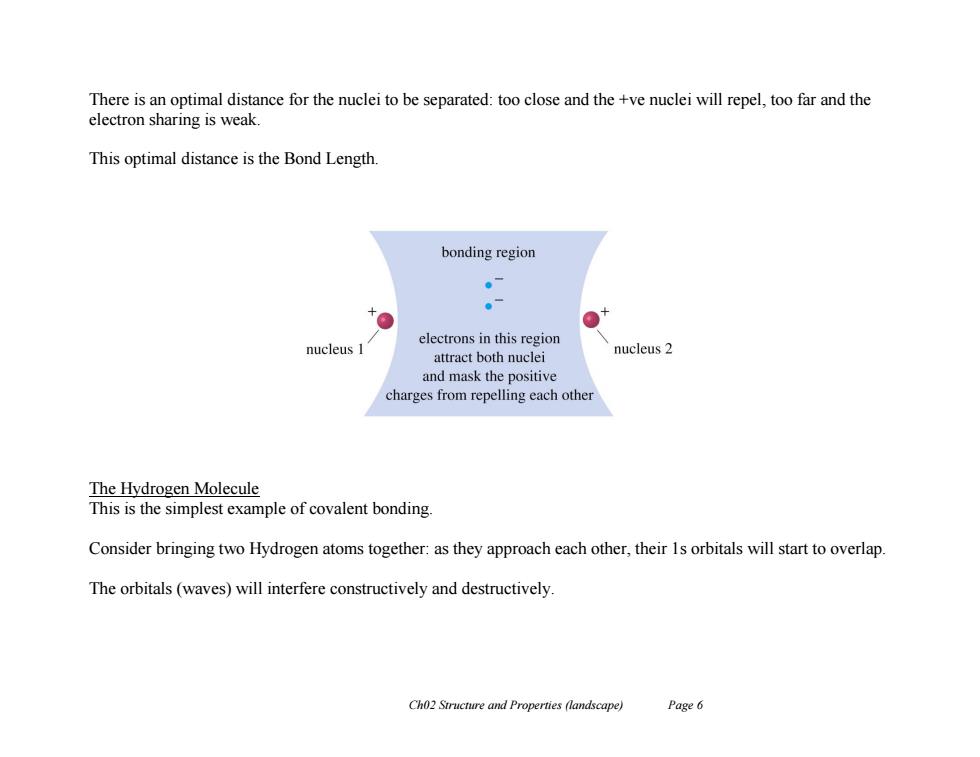
There is an optimal distance for the nuclei to be separated:too close and the +ve nuclei will repel,too far and the electron sharing is weak. This optimal distance is the Bond Length bonding region 。7 。 electrons in this region nucleus 1 attract both nuclei nucleus 2 and mask the positive charges from repelling each other The Hydrogen Molecule This is the simplest example of covalent bonding. Consider bringing two Hydrogen atoms together:as they approach each other,their 1s orbitals will start to overlap. The orbitals(waves)will interfere constructively and destructively Ch02 Structure and Properties (landscape) Page 6 Ch02 Structure and Properties (landscape) Page 6 There is an optimal distance for the nuclei to be separated: too close and the +ve nuclei will repel, too far and the electron sharing is weak. This optimal distance is the Bond Length. The Hydrogen Molecule This is the simplest example of covalent bonding. Consider bringing two Hydrogen atoms together: as they approach each other, their 1s orbitals will start to overlap. The orbitals (waves) will interfere constructively and destructively