正在加载图片...
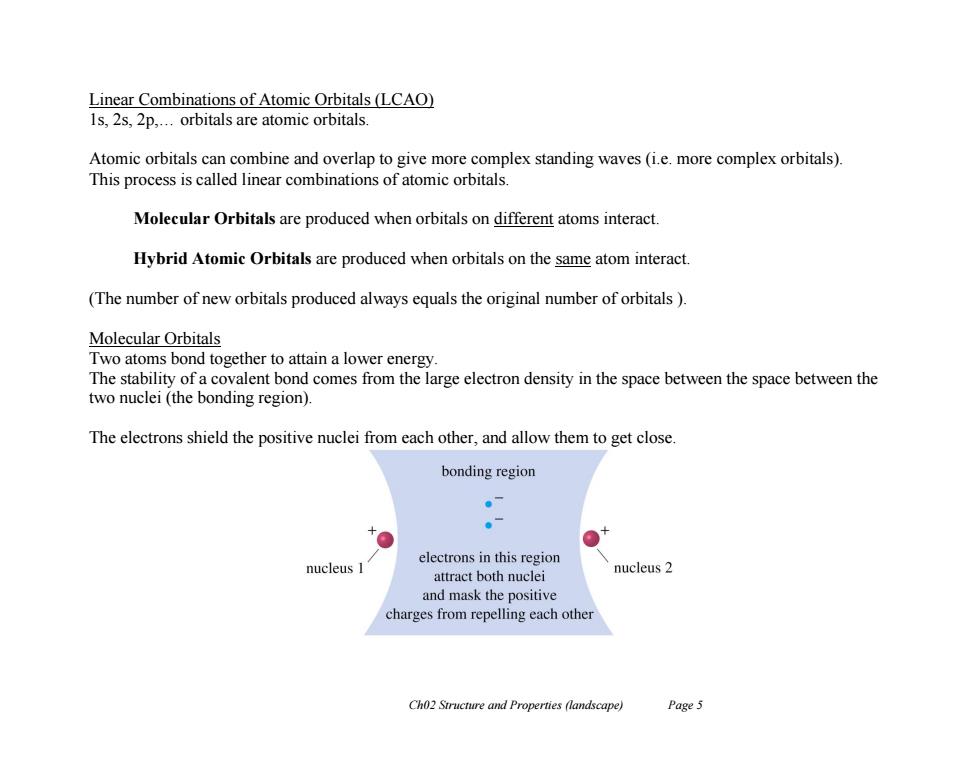
Linear Combinations of Atomic Orbitals(LCAO) 1s,2s,2p,...orbitals are atomic orbitals. Atomic orbitals can combine and overlap to give more complex standing waves (i.e.more complex orbitals). This process is called linear combinations of atomic orbitals. Molecular Orbitals are produced when orbitals on different atoms interact. Hybrid Atomic Orbitals are produced when orbitals on the same atom interact. (The number of new orbitals produced always equals the original number of orbitals ) Molecular Orbitals Two atoms bond together to attain a lower energy. The stability of a covalent bond comes from the large electron density in the space between the space between the two nuclei (the bonding region) The electrons shield the positive nuclei from each other,and allow them to get close. bonding region 。7 electrons in this region nucleus I attract both nuclei nucleus 2 and mask the positive charges from repelling each other Ch02 Structure and Properties (landscape) Page 5Ch02 Structure and Properties (landscape) Page 5 Linear Combinations of Atomic Orbitals (LCAO) 1s, 2s, 2p,… orbitals are atomic orbitals. Atomic orbitals can combine and overlap to give more complex standing waves (i.e. more complex orbitals). This process is called linear combinations of atomic orbitals. Molecular Orbitals are produced when orbitals on different atoms interact. Hybrid Atomic Orbitals are produced when orbitals on the same atom interact. (The number of new orbitals produced always equals the original number of orbitals ). Molecular Orbitals Two atoms bond together to attain a lower energy. The stability of a covalent bond comes from the large electron density in the space between the space between the two nuclei (the bonding region). The electrons shield the positive nuclei from each other, and allow them to get close