正在加载图片...
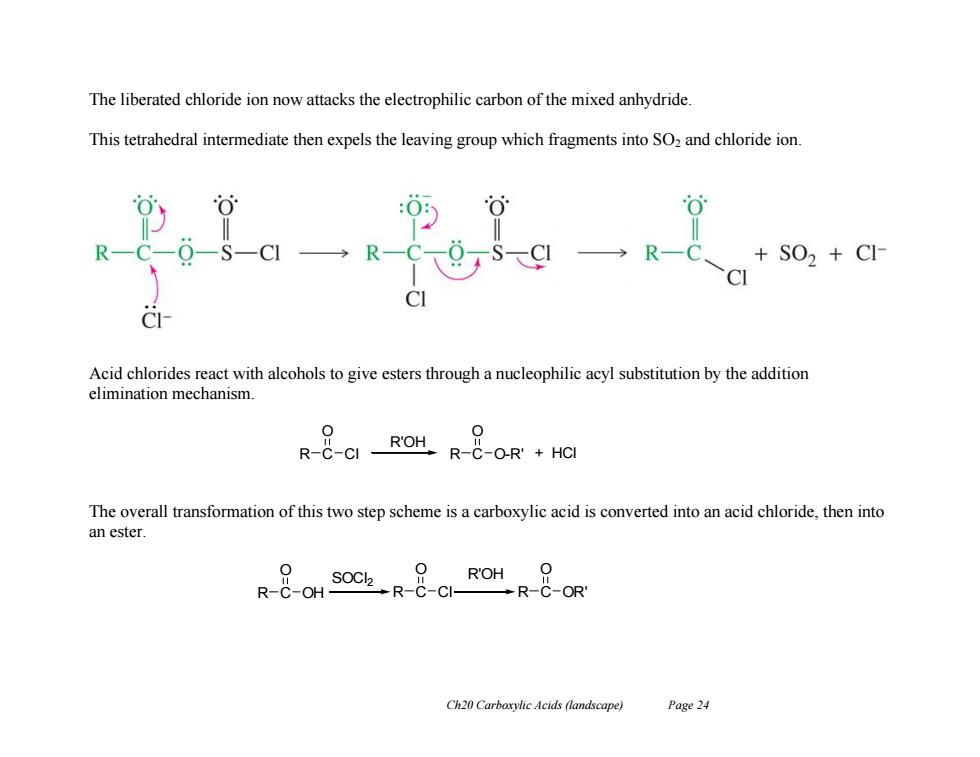
The liberated chloride ion now attacks the electrophilic carbon of the mixed anhydride This tetrahedral intermediate then expels the leaving group which fragments into SO2 and chloride ion. →RC +S02+C1 C Acid chlorides react with alcohols to give esters through a nucleophilic acyl substitution by the addition elimination mechanism 0 0 R--CI ROH.HCI The overall transformation of this two step scheme is a carboxylic acid is converted into an acid chloride,then into an ester. OCl2 R'OH 0 R-C-OH- R-C-CI- R-C-OR' Ch20 Carboxylic Acids (landscape) Page 24 Ch20 Carboxylic Acids (landscape) Page 24 The liberated chloride ion now attacks the electrophilic carbon of the mixed anhydride. This tetrahedral intermediate then expels the leaving group which fragments into SO2 and chloride ion. Acid chlorides react with alcohols to give esters through a nucleophilic acyl substitution by the addition elimination mechanism. The overall transformation of this two step scheme is a carboxylic acid is converted into an acid chloride, then into an ester. R C O Cl R C O O-R' R'OH + HCl R C O OH R C O Cl R C O OR' SOCl2 R'OH