正在加载图片...
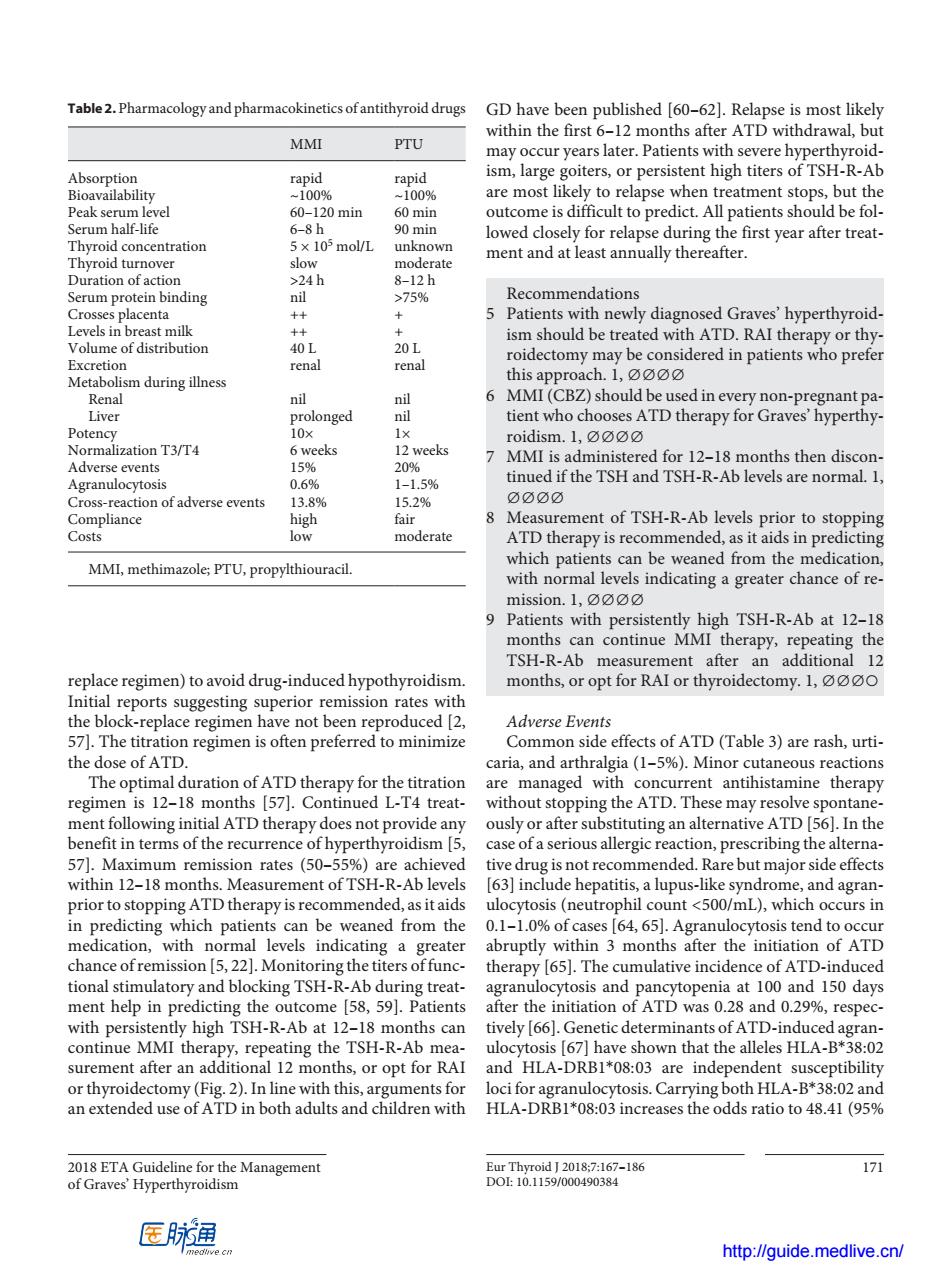
Table2.Pharmacologyand pharmacokineticsofantithyroid drugs GD have been published [60-62).Relapse is most likely within the first 6-12 months after ATD withdrawal.bu MMI PTU may occur years later.Patients with severe hyperthyroid- ism,large goiters,or persistent high titers of TSH-R-Ab rapid are most likely to relapse when treatment stops,but the 20 min outcome is difficult to predict.All patients should be fol TthroidconcEatratian 5×10mol/L 8-12h 75% mendation 5 Patients with newly diagnosed Graves'hyperthyroid- 40 ism should be treated with ATD.RAI ther py or thy 20L rena renal roidectomy may be considered in patients who prefer daring ne this approach.1,0 6 MMI(CBZ)should be used in every non-pregnant pa 三m olonged nt who chooses ATD therapy for Graves hyperthy eeks is red for 12-18 s then 1-1.5% TSH ndsc aRaa CrosIa ion of adverse events 52% Measurement of TSH-R-Ab levels prior to stopping low moderate ATD therapy is recommended,as it aids in predicting MMI,methimazole PTU,propylthiouracil. which patients can be weaned from the medication with normal levels indicating a greater chance of re. mission.1, Patients with pe TSH-R -Ab at 12. TSH-R-Ab replace regimen)to avoid drug-induced hypothyroidisn or opt for RAI roider omy.1,0o0 ing su rior remission rates with the blrepacrm have not been reproduced Adverse Events 57].The titration regimen is often preferred to minimize Common side effects of ATD(Table 3)are rash,urti- the dose of ATD. caria,and arthralgia(1-5%).Minor cutaneous reactions regime 57) are managed witl trea se n ATD th rapy oes n pro alle ates (50-556 ide effect within 12-18 months.Measurement of TSH-R-Ab levels 63]include hepatitis.a lupus-like syndrome.and ag ran. prior to stopping ATD therapy is recommended,as itaids ulocytosis(neutrophil count <500/mL),which occurs in in predicting which patients can be weaned from the 0.1-1.0%of cases [64,65].Agranulocytosis tend to occur medication,with normal abruptly within 3 months after the initiation of ATD chance ofre ission ers offunc- atory and blo therapy [65].The cumulative incidence of ATD- cking TSH sis and iTSH-RA A78opeaat100 150 day espe 11g tinue MMI the h y9 the TSH-R-Ab (671 hav tha surement after an 1 months or opt for RAI and HLA-DRB1*08:03 are inder endent suscentibility am3noaaaaam ments for loci for a anulocytosis.Carrying both HLA-B*38:02 and HLA-DRB1*08:03 increases the odds ratio to 48.41(95% 171 医肺润 http://quide medlive.cn/2018 ETA Guideline for the Management of Graves’ Hyperthyroidism Eur Thyroid J 2018;7:167–186 171 DOI: 10.1159/000490384 replace regimen) to avoid drug-induced hypothyroidism. Initial reports suggesting superior remission rates with the block-replace regimen have not been reproduced [2, 57]. The titration regimen is often preferred to minimize the dose of ATD. The optimal duration of ATD therapy for the titration regimen is 12–18 months [57]. Continued L-T4 treatment following initial ATD therapy does not provide any benefit in terms of the recurrence of hyperthyroidism [5, 57]. Maximum remission rates (50–55%) are achieved within 12–18 months. Measurement of TSH-R-Ab levels prior to stopping ATD therapy is recommended, as it aids in predicting which patients can be weaned from the medication, with normal levels indicating a greater chance of remission [5, 22]. Monitoring the titers of functional stimulatory and blocking TSH-R-Ab during treatment help in predicting the outcome [58, 59]. Patients with persistently high TSH-R-Ab at 12–18 months can continue MMI therapy, repeating the TSH-R-Ab measurement after an additional 12 months, or opt for RAI or thyroidectomy (Fig. 2). In line with this, arguments for an extended use of ATD in both adults and children with GD have been published [60–62]. Relapse is most likely within the first 6–12 months after ATD withdrawal, but may occur years later. Patients with severe hyperthyroidism, large goiters, or persistent high titers of TSH-R-Ab are most likely to relapse when treatment stops, but the outcome is difficult to predict. All patients should be followed closely for relapse during the first year after treatment and at least annually thereafter. Recommendations 5 Patients with newly diagnosed Graves’ hyperthyroidism should be treated with ATD. RAI therapy or thyroidectomy may be considered in patients who prefer this approach. 1, ∅∅∅∅ 6 MMI (CBZ) should be used in every non-pregnant patient who chooses ATD therapy for Graves’ hyperthyroidism. 1, ∅∅∅∅ 7 MMI is administered for 12–18 months then discontinued if the TSH and TSH-R-Ab levels are normal. 1, ∅∅∅∅ 8 Measurement of TSH-R-Ab levels prior to stopping ATD therapy is recommended, as it aids in predicting which patients can be weaned from the medication, with normal levels indicating a greater chance of remission. 1, ∅∅∅∅ 9 Patients with persistently high TSH-R-Ab at 12–18 months can continue MMI therapy, repeating the TSH-R-Ab measurement after an additional 12 months, or opt for RAI or thyroidectomy. 1, ∅∅∅○ Adverse Events Common side effects of ATD (Table 3) are rash, urticaria, and arthralgia (1–5%). Minor cutaneous reactions are managed with concurrent antihistamine therapy without stopping the ATD. These may resolve spontaneously or after substituting an alternative ATD [56]. In the case of a serious allergic reaction, prescribing the alternative drug is not recommended. Rare but major side effects [63] include hepatitis, a lupus-like syndrome, and agranulocytosis (neutrophil count <500/mL), which occurs in 0.1–1.0% of cases [64, 65]. Agranulocytosis tend to occur abruptly within 3 months after the initiation of ATD therapy [65]. The cumulative incidence of ATD-induced agranulocytosis and pancytopenia at 100 and 150 days after the initiation of ATD was 0.28 and 0.29%, respectively [66]. Genetic determinants of ATD-induced agranulocytosis [67] have shown that the alleles HLA-B*38:02 and HLA-DRB1*08:03 are independent susceptibility loci for agranulocytosis. Carrying both HLA-B*38:02 and HLA-DRB1*08:03 increases the odds ratio to 48.41 (95% Table 2. Pharmacology and pharmacokinetics of antithyroid drugs MMI PTU Absorption rapid rapid Bioavailability ~100% ~100% Peak serum level 60–120 min 60 min Serum half-life 6–8 h 90 min Thyroid concentration 5 × 105 mol/L unknown Thyroid turnover slow moderate Duration of action >24 h 8–12 h Serum protein binding nil >75% Crosses placenta ++ + Levels in breast milk ++ + Volume of distribution 40 L 20 L Excretion renal renal Metabolism during illness Renal nil nil Liver prolonged nil Potency 10× 1× Normalization T3/T4 6 weeks 12 weeks Adverse events 15% 20% Agranulocytosis 0.6% 1–1.5% Cross-reaction of adverse events 13.8% 15.2% Compliance high fair Costs low moderate MMI, methimazole; PTU, propylthiouracil. http://guide.medlive.cn/