正在加载图片...
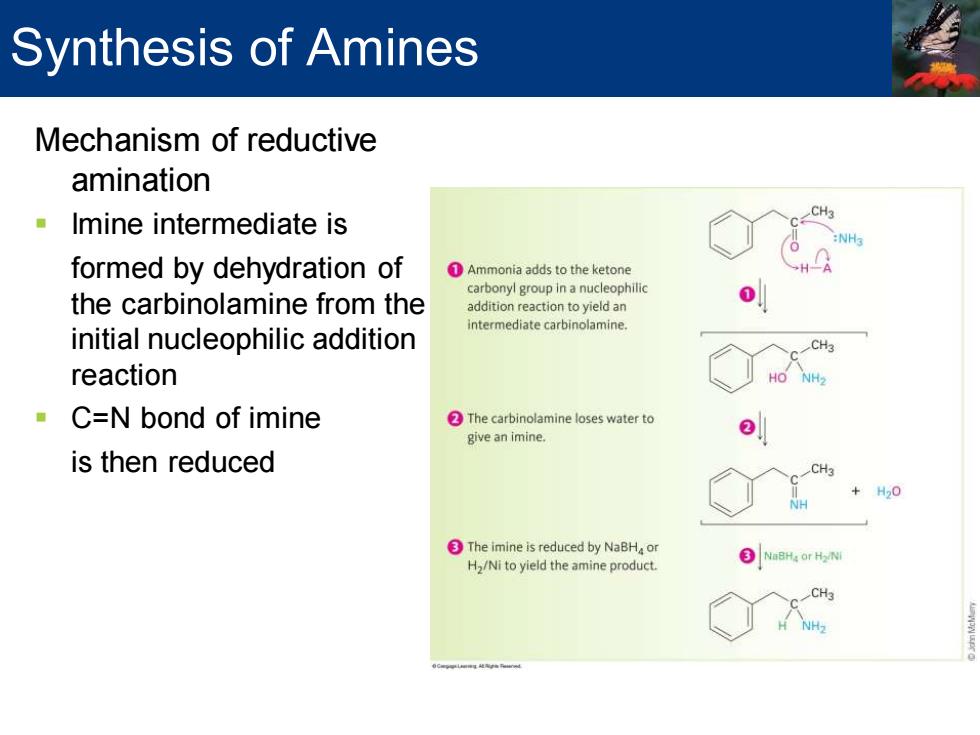
Synthesis of Amines Mechanism of reductive amination Imine intermediate is CH3 formed by dehydration of 1Ammonia adds to the ketone carbonyl group in a nucleophilic the carbinolamine from the addition reaction to yield an intermediate carbinolamine. initial nucleophilic addition CH3 reaction HO NH2 C=N bond of imine The carbinolamine loses water to give an imine. is then reduced CH3 +H20 The imine is reduced by NaBHa or 3 NaBHa or Hy/N H2/Ni to yield the amine product. CH3 NH2Mechanism of reductive amination ▪ Imine intermediate is formed by dehydration of the carbinolamine from the initial nucleophilic addition reaction ▪ C=N bond of imine is then reduced Synthesis of Amines