正在加载图片...
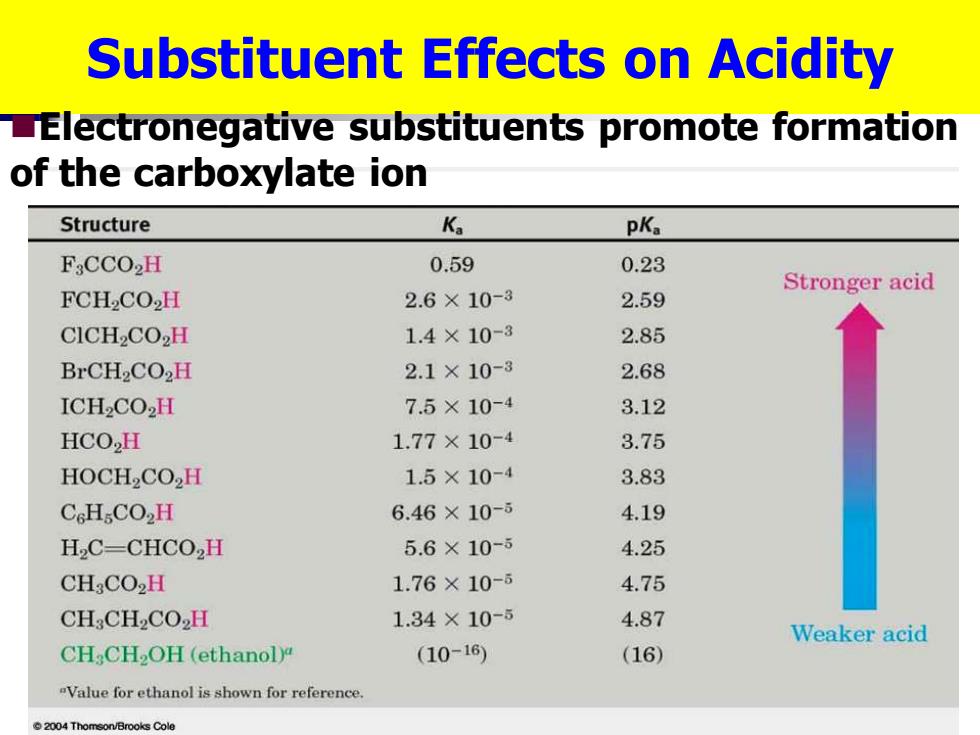
Substituent Effects on Acidity LElectronegative substituents promote formation of the carboxylate ion Structure K pKa F:CCO2H 0.59 0.23 Stronger acid FCH2CO2H 2.6×10-3 2.59 CICH2CO2H 1.4×10-3 2.85 BrCH2CO2H 2.1×10-3 2.68 ICH2CO2H 7.5×10-4 3.12 HCO,H 1.77×10-4 3.75 HOCH2CO2H 1.5×10-4 3.83 CcHCO2H 6.46×10-ǒ 4.19 H,C-CHCO,H 5.6×10-5 4.25 CH:CO2H 1.76×10-6 4.75 CH:CH2CO2H 1.34×10-5 4.87 Weaker acid CH CH2OH(ethanol) (10-16) (16) "Value for ethanol is shown for reference 004 Thomson/Brooks ColeSubstituent Effects on Acidity ◼Electronegative substituents promote formation of the carboxylate ion