正在加载图片...
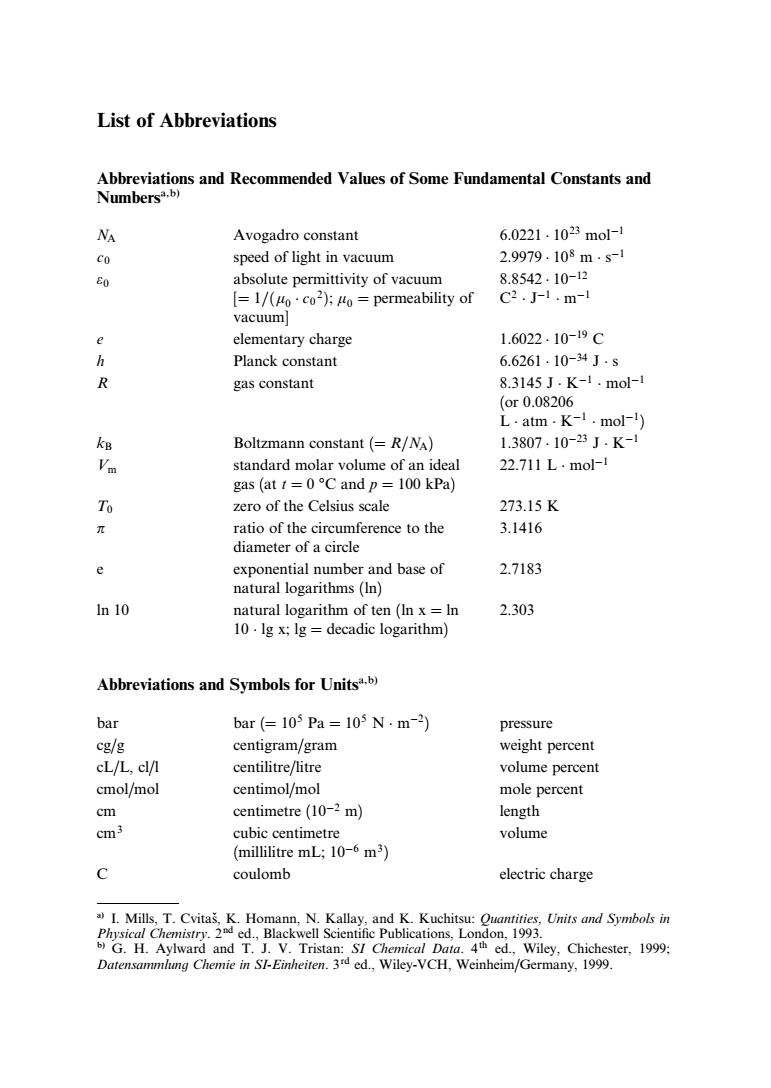
List of Abbreviations Abbreviations and Recommended Values of Some Fundamental Constants and NA Avogadro constant 6.0221.1023mol-1 speed of light in vacuum 2.9979.108ms- absolute permittivity of vacuum 8.8542.10-12 [=1/(Hoco2):Ho =permeability of C2.J-1.m-1 vacuum] elementary charge 1.6022.10-19C h Planck constant 6.6261.10-34J.s R gas constant 8.3145J.K-1.mol-l (or0.08206 L.atm.K-1.mol-) Boltzmann constant (R/NA) 1.3807.10-23J.K- standard molar volume of an ideal 227111.m0l- gas (att=0C and p=100 kPa) ero of the Celsius scale 273.15K ratio of the circumference to the 3.1416 diameter of a circle e 27183 In 10 natural logarithm of ten(In x=In 2.303 10.lg x:lg decadic logarithm) Abbreviations and Symbols for Unitsb) bar bar(=105Pa=105N.m-2 pressure Cg/g centigram/gram weight percent cL/L,cl/ centilitre/litre volume percen cmol/mol centimol/mol mole percent cm centimetre(10-2m) length Cm3 cubic centimetre volume (millilitre mL;10-6 m3) c coulomb electric charge SI C nical Daig a ed Wiley,Chichester,1999: Datensammlung Chemie in SI-Einheiten.3d ed.,Wiley-VCH,Weinheim/Germany,1999. List of Abbreviations Abbreviations and Recommended Values of Some Fundamental Constants and Numbersa,b) NA Avogadro constant 6:0221 1023 mol1 c0 speed of light in vacuum 2:9979 108 m s1 e0 absolute permittivity of vacuum [¼ 1=ðm0 c0 2Þ; m0 ¼ permeability of vacuum] 8:8542 1012 C2 J1 m1 e elementary charge 1:6022 1019 C h Planck constant 6:6261 1034 J s R gas constant 8.3145 J K1 mol1 (or 0.08206 L atm K1 mol1) kB Boltzmann constant (¼ R=NA) 1:3807 1023 J K1 Vm standard molar volume of an ideal gas (at t ¼ 0 C and p ¼ 100 kPa) 22.711 L mol1 T0 zero of the Celsius scale 273.15 K p ratio of the circumference to the diameter of a circle 3.1416 e exponential number and base of natural logarithms (ln) 2.7183 ln 10 natural logarithm of ten (ln x ¼ ln 10 lg x; lg ¼ decadic logarithm) 2.303 Abbreviations and Symbols for Unitsa,b) bar bar (¼ 105 Pa ¼ 105 N m2) pressure cg/g centigram/gram weight percent cL/L, cl/l centilitre/litre volume percent cmol/mol centimol/mol mole percent cm centimetre (102 m) length cm3 cubic centimetre (millilitre mL; 106 m3) volume C coulomb electric charge a) I. Mills, T. Cvitasˇ, K. Homann, N. Kallay, and K. Kuchitsu: Quantities, Units and Symbols in Physical Chemistry. 2nd ed., Blackwell Scientific Publications, London, 1993. b) G. H. Aylward and T. J. V. Tristan: SI Chemical Data. 4th ed., Wiley, Chichester, 1999; Datensammlung Chemie in SI-Einheiten. 3rd ed., Wiley-VCH, Weinheim/Germany, 1999.������������������