正在加载图片...
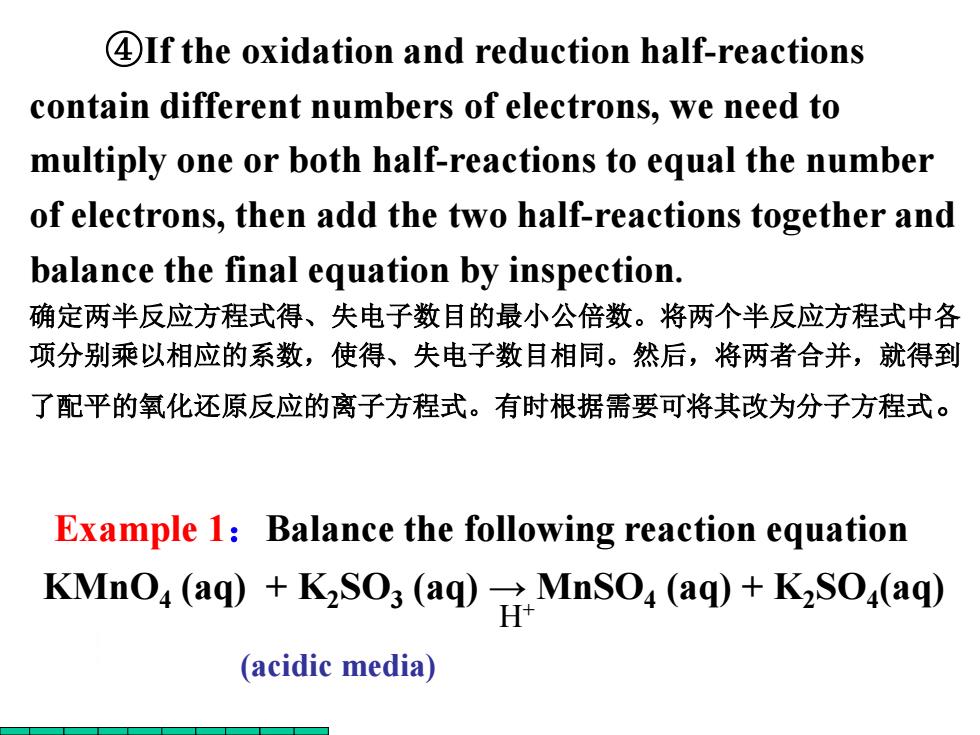
4If the oxidation and reduction half-reactions contain different numbers of electrons,we need to multiply one or both half-reactions to equal the number of electrons,then add the two half-reactions together and balance the final equation by inspection. 确定两半反应方程式得、失电子数目的最小公倍数。将两个半反应方程式中各 项分别乘以相应的系数,使得、失电子数目相同。然后,将两者合并,就得到 了配平的氧化还原反应的离子方程式。有时根据需要可将其改为分子方程式。 Example 1:Balance the following reaction equation KMnO,(aq)+KSO;(aq)MnSO,(aq)+KSO,(aq) (acidic media)Example 1:Balance the following reaction equation ④If the oxidation and reduction half-reactions contain different numbers of electrons, we need to multiply one or both half-reactions to equal the number of electrons, then add the two half-reactions together and balance the final equation by inspection. 确定两半反应方程式得、失电子数目的最小公倍数。将两个半反应方程式中各 项分别乘以相应的系数,使得、失电子数目相同。然后,将两者合并,就得到 了配平的氧化还原反应的离子方程式。有时根据需要可将其改为分子方程式。 KMnO4 (aq) + K2SO3 (aq) → MnSO4 (aq) + K2SO4 (aq) H+ (acidic media)