正在加载图片...
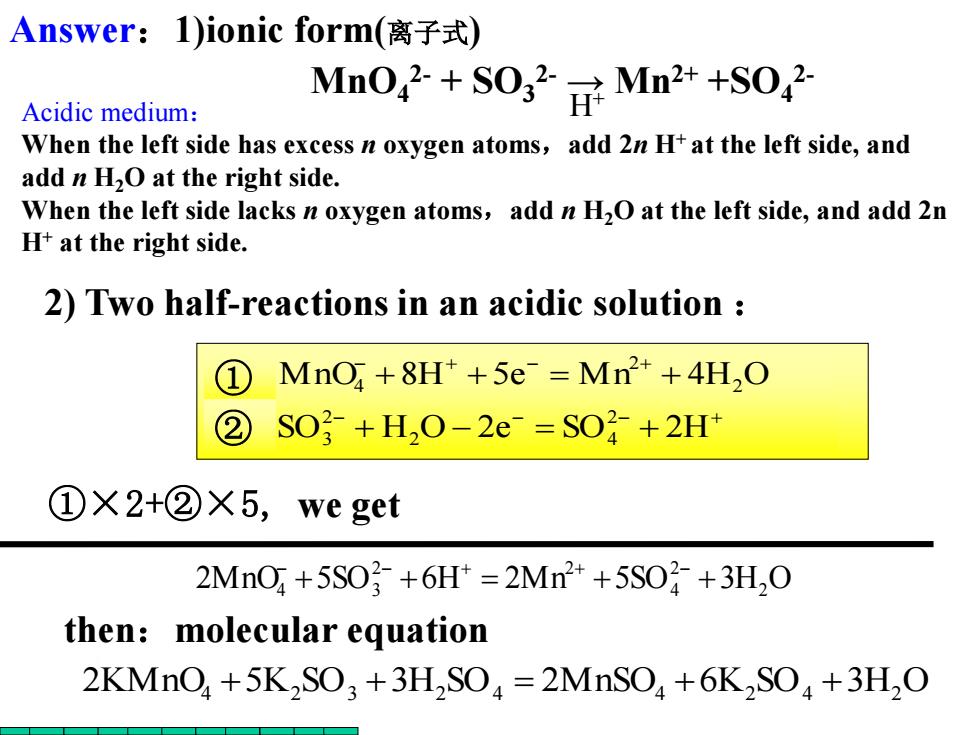
Answer:1)ionic form(离子式) Acidic medium: Mn042+S0g2NMn2++s042 When the left side has excess n oxygen atoms,add 2n H+at the left side,and add n H2O at the right side. When the left side lacks n oxygen atoms,add n H2O at the left side,and add 2n H+at the right side. 2)Two half-reactions in an acidic solution ① MnO,+8H*+5e Mn+4H,O ② SO2+H,O-2e-=SO+2H+ ①×2+②X5,we get 2MnO+5S03+6H=2Mn2++5SO子+3H,0 then:molecular equation 2KMnO+5K2SO3+3H,SO=2MnSO+6K2SO+3H,O + + + + + + + + SO H O 2e SO 2H MnO 8H 5e M n 4H O 2 2 4 2 3 2 2 ① 4 ② ①×2+②×5, we get 2MnO 5SO 6H 2Mn 5SO 3H2 O 2 4 2 2 4 + 3 + + + + + 2) Two half-reactions in an acidic solution : 2KMnO4 +5K2 SO3 +3H2 SO4 2MnSO4 +6K2 SO4 +3H2 O then:molecular equation MnO4 2- + SO3 2- → Mn2+ +SO4 2- H+ Answer:1)ionic form(离子式) Acidic medium: When the left side has excess n oxygen atoms,add 2n H+ at the left side, and add n H2O at the right side. When the left side lacks n oxygen atoms,add n H2O at the left side, and add 2n H+ at the right side