正在加载图片...
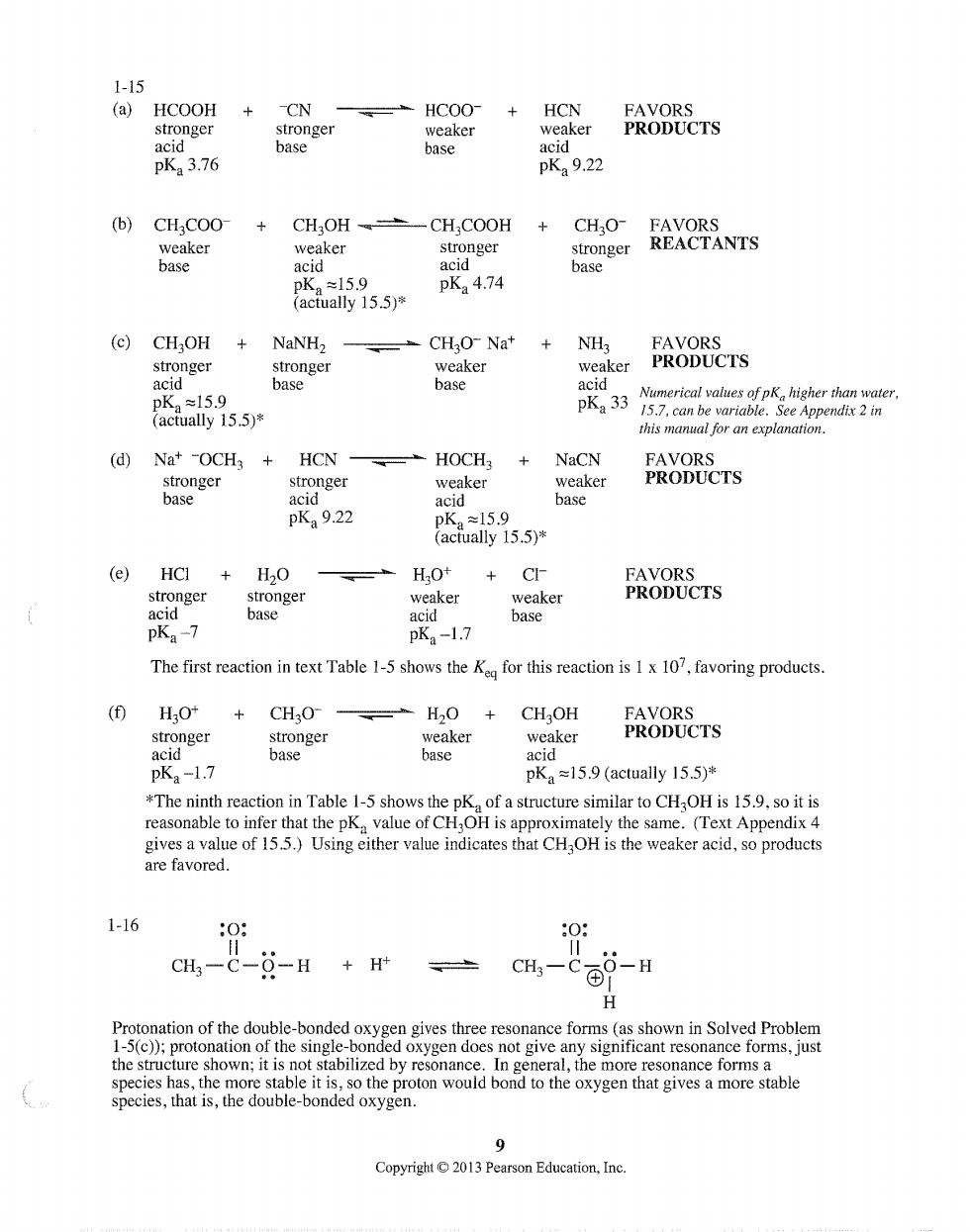
1-15 (a)HCOOH +CN =HC00 +HCN weaker PRODUCTS pK3.76 pKa 9.22 (b)CH;COO- -CH COOH CHO- stronger stronger REACTANTS ✉15.9 K4.74 (actually 15.5)* (c)CH3OH NaNHz CHO-Nat NH3 FAVORS stronger weaker weaker PRODUCTS base base 15.9 ctually 155)* this manal for an explanation. (d)Nat -OCH3 HCN --HOCH3 NaCN FAVORS stronger stronger weaker weaker PRODUCTS base acid acid base pKa9.22 (e)HCI+H2O CI- FAVORS stronger stronger weaker weaker PRODUCTS base acid base pKa-7 pKa-1.7 The first reaction in text Table 1-5 shows theK for this reaction is 1x 107,favoring products. (f)HO +CHO- =H20+ CHOH PRODUCTS base acid pKa-1.7 pKa =15.9 (actually 15.5)* *The ninth reaction in Table 1-5 shows the pKa of a structure similar to CHOH is 15.9.so it is reasonable to infer hat the pKa value of CH,OH is approximately the same.(Text Appendix 4 gives a value of 15.5.)Using either value indicates that CH OH is the weaker acid,so products are favored. 1-16 0 0 CH-C-0-H +H+ H ation of the double the structure shown:it ispot stabso the protoo would pond ne the monhesonance foomsable the oxygen that gives a more stable Copyright 9 2013 Pearson Education,Inc