
Chapter/16Carbohydrates
Chapter 16 Carbohydrates

Carbohydrates are sugars andtheir derivatives. The commonsugars contain an aldehyde orketone carbonyl group as well asa number of hydroxy groups
Carbohydrates are sugars and their derivatives. The common sugars contain an aldehyde or ketone carbonyl group as well as a number of hydroxy groups
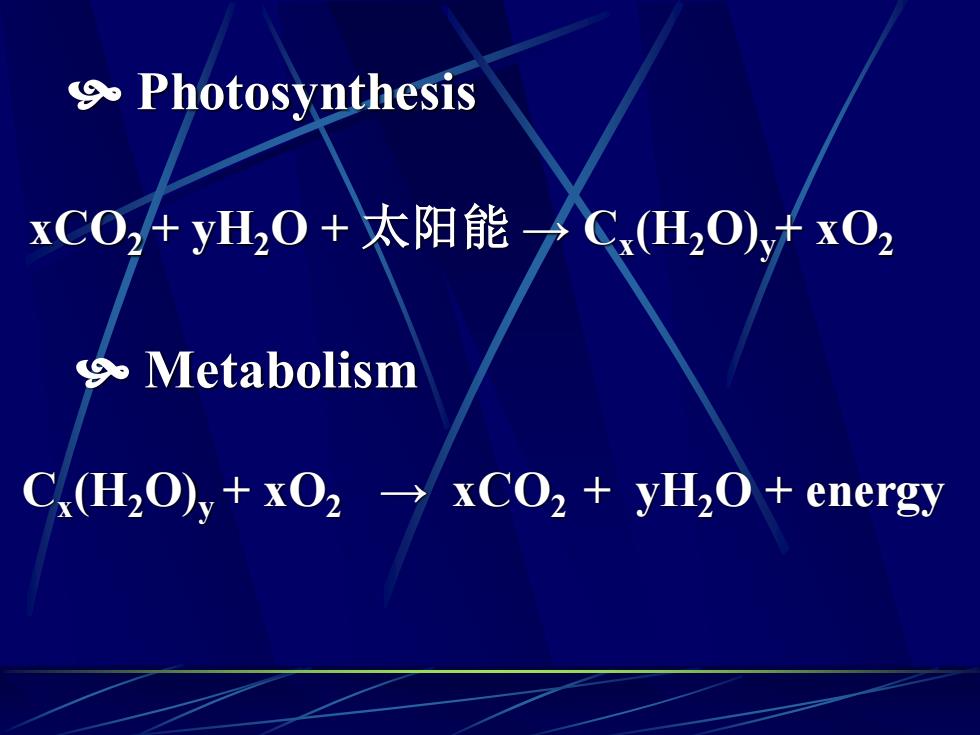
9 PhotosynthesisxCO,+yH,0+太阳能→ C(H,O)+ xO29. MetabolismC(H,O),+ xO2 → xCO2 + yH,O + energy
Photosynthesis xCO2 + yH2O + 太阳能 → Cx (H2O)y+ xO2 Cx (H2O)y + xO2 → xCO2 + yH2O + energy Metabolism
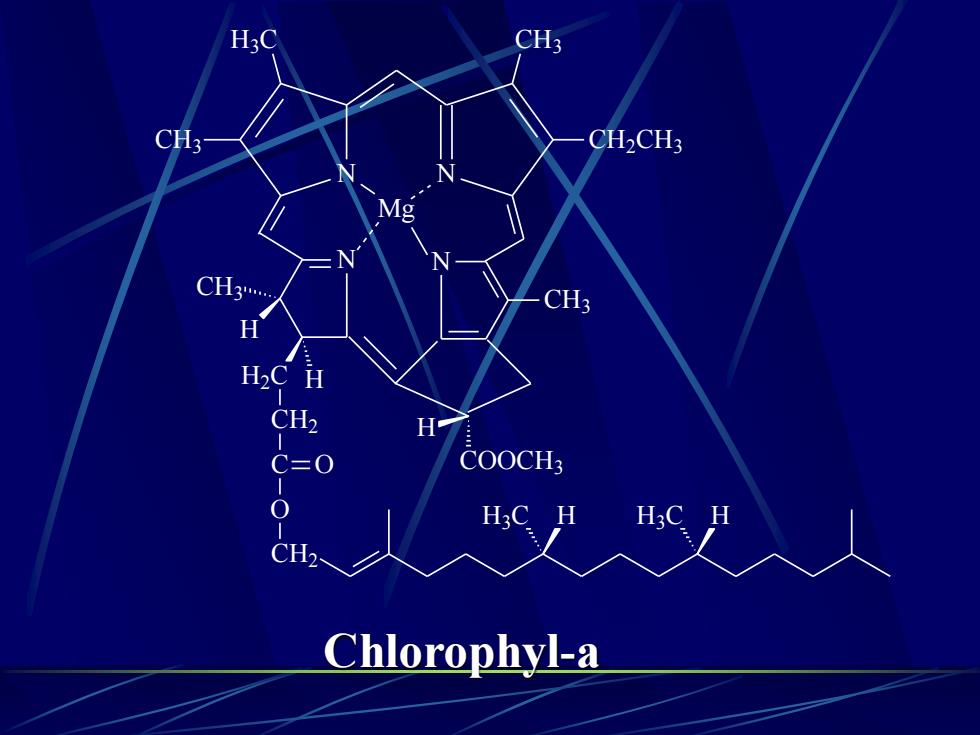
H3CCH3CH3CH2CH3MgNCH.CH3HH2CHCH2HCOOCH3C=00H3C.HH3CHCH2Chlorophyl-a
N CH3 H COOCH3 N H H2C CH3 H CH2 C O O CH2 H3C H H3C H N Mg N CH3 CH2CH3 H3C CH3 Chlorophyl-a
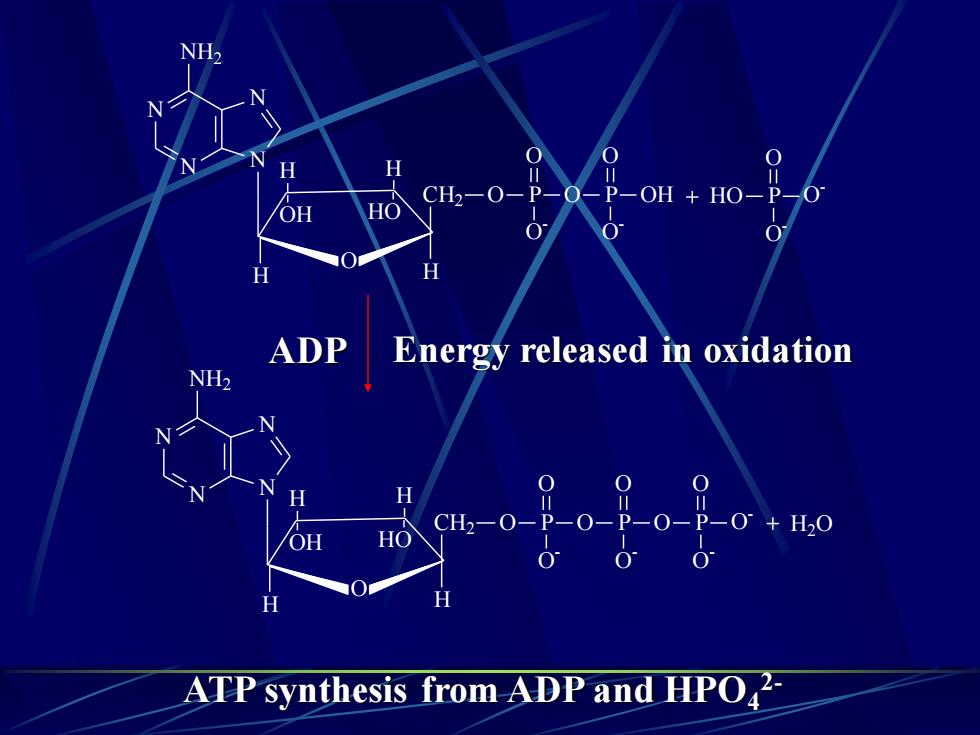
NH2N0O0HH0CH2-O-P-O-P-OH +HO-P-HOOH0'00HHADPEnergy released in oxidationNH20=O=0=HP--P-0° + H20CH2-0-P-0--0-HOOH0.00HHATPsynthesisfromADPandHPO2
HO P O - O - O N N N N NH2 H O OH H HO H CH2 O P O P OH O - O - O O H H2O N N N N NH2 H O OH H HO H CH2 O P O P O O - O - O O P O - O - O H ADP Energy released in oxidation ATP synthesis from ADP and HPO4 2-
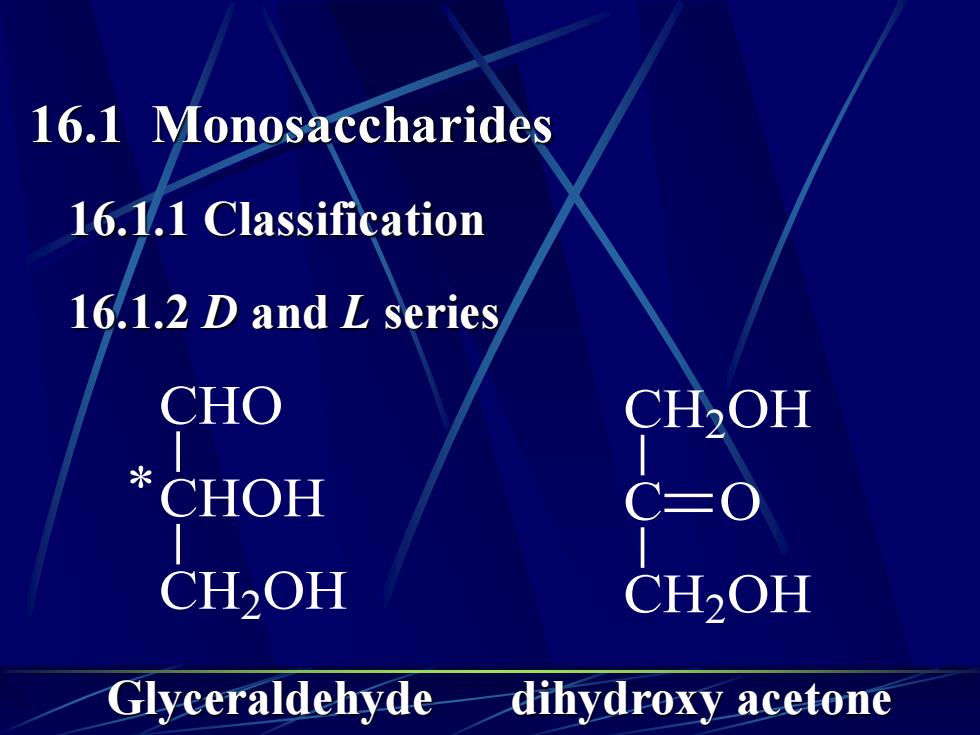
16.1 Monosaccharides16.1.1Classification16.1.2 D and L seriesCHOCH,OH*CHOHC=0CH2OHCH2OHGlyceraldehydedihydroxy acetone
16.1 Monosaccharides 16.1.1 Classification 16.1.2 D and L series C CH2 OH O CH2 OH CHOH CH2 OH CHO * Glyceraldehyde dihydroxy acetone

OO三IC-HC-H℃-OHHO-℃-HHCH2OHCH2OHD-(+)-glyceraldehydeL-(-)-glyceraldehydeR-(+)-glyceraldehydeS-(-)-glyceraldehyde
C H O C CH2 OH H OH C H O C CH2 OH HO H D-(+)-glyceraldehyde L-(-)-glyceraldehyde R-(+)-glyceraldehyde S-(-)-glyceraldehyde

CHOCHOH-C-OHH-C-OHHO-C-HHO-C-HH-C-OHH-C-OHH-C-OHH-C-OHCH2OHCH,OH飞楔式a5Fischer投影式b、CH2OHCH.OH-0HHOHOHHHOHH16.1.3OHHOHOHHOHHOHHOHStructureHaworthHOH2CHOH,COHOHOOHHOHOOHOHOHβ-D-(+)-葡萄糖α-D-(+)-葡萄糖
16.1.3 Structure CHO C C C C CH2OH OH H OH H OH H HO H CHO C C C C CH2OH OH H OH H OH H HO H O H H OH OH H OH CH2OH OH H H O OH H OH OH H CH2OH H OH H H O HO HO HOH2C OH OH O HO HO HOH2C OH OH a 、飞楔式 b、Fischer投影式 c d e f -D-(+)-葡萄糖 -D-(+)-葡萄糖 Haworth
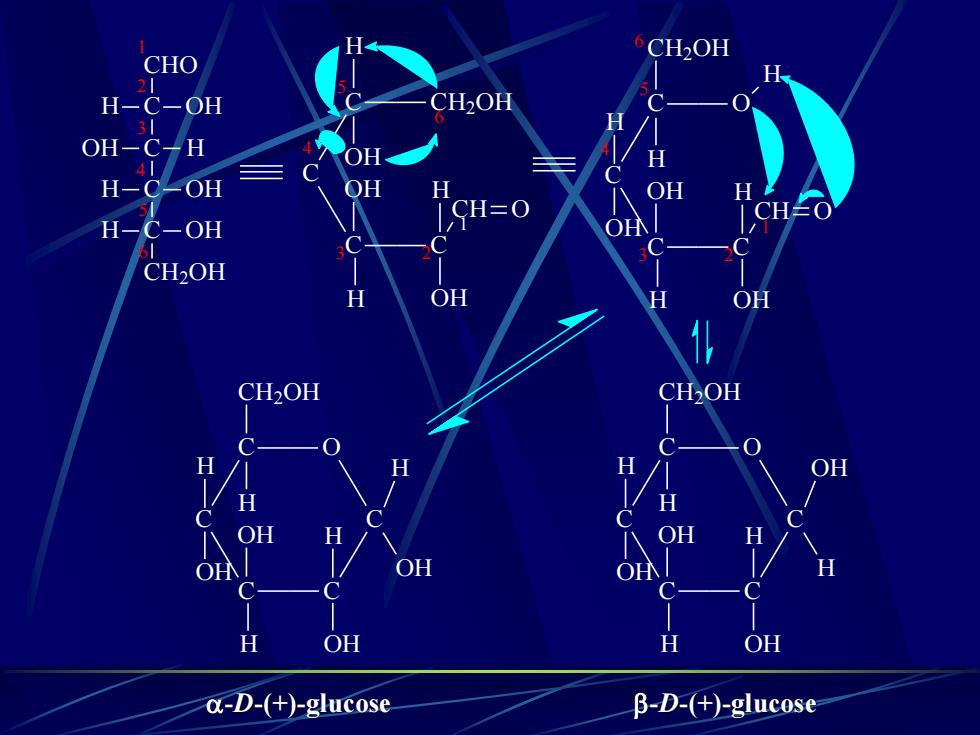
CH2OHCHOH0CH2OHH-C-OH31OH-C-HOHH41H-C-OHOHHOHHLLCHOCH=OOH/1H-C-OHCCCCH,OHHOHHOHCH2OHCH,OH00HHHOHHH福OHHOHHOHHOHOHNCHHOHOHα-D-(+)-glucoseβ-D-(+)-glucose
C OH C CHO H C H C OH OH CH2OH OH H H 1 2 3 4 5 6 C C CH2OH C C CH O H OH OH H OH H 1 3 2 4 5 6 C C O C C CH O H CH2OH H OH H OH H 1 3 2 4 5 6 H OH C C O C C C CH2OH H OH H OH H H OH H OH C C O C C C CH2OH H OH H OH H H OH OH H -D-(+)-glucose -D-(+)-glucose
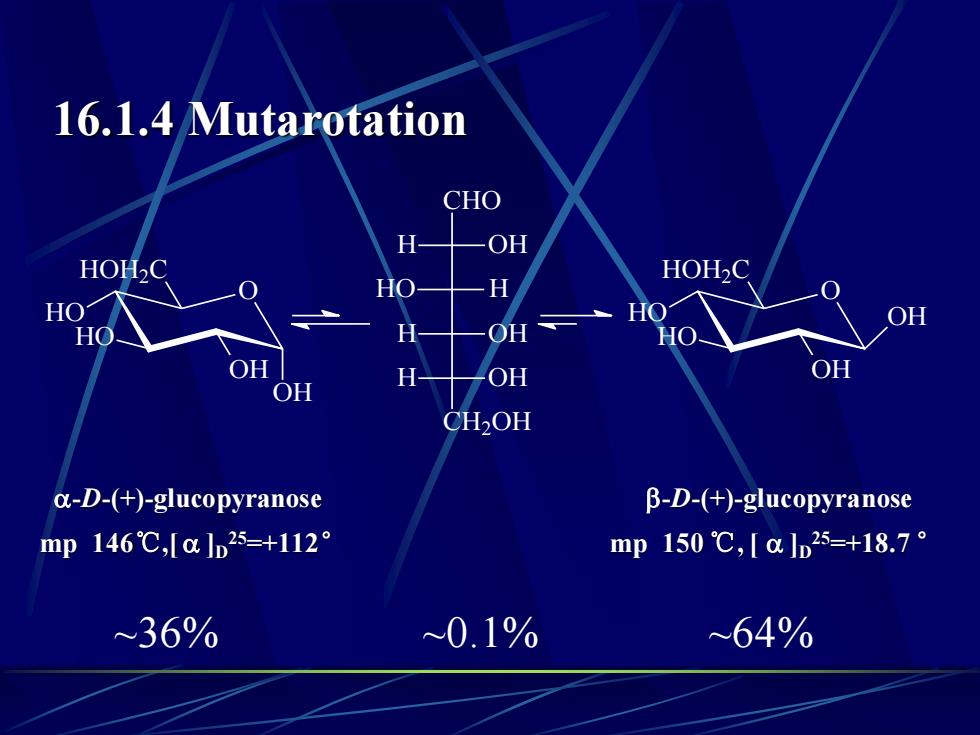
16.1.4 MutarotationCHOHOHHOH2CHOH2CHHO0OHOHOOHHOHHOHOOHOHHOHOHCH2OHα-D-(+)-glucopyranoseβ-D-(+)-glucopyranosemp 150 ℃,[α lb25=+18.7 °mp 146℃,[α lb25=+112°~0.1%~64%~36%
16.1.4 Mutarotation O HO HO OH HOH2C OH O HO HO OH HOH2C OH CHO H OH HO H H OH CH2OH H OH -D-(+)-glucopyranose -D-(+)-glucopyranose mp 146℃,[ ]D 25=+112° mp 150 ℃, [ ]D 25=+18.7 ° ~36% ~0.1% ~64%