
Chapter 3. Alkenes
Chapter 3. Alkenes

单烯烃是指分子中含有一个碳碳双键(C=C)的不饱和开链烃。单烯烃的通式是C,H2n.烯键是烯?烃的官能团
单烯烃是指分子中含有一个碳碳 双键(C=C)的不饱和开链烃。 单烯烃的通式是CnH2n, 烯键是烯 烃的官能团

L StructureofAlkenes从键能上看,C-C键的能为345.6kJ/mol,而C=C键的键能为610kJ/mol,光谱和电子衍射测定证明乙烯的平面结构如下:
1 Structure of Alkenes 从键能上看,C-C键的能为345.6kJ/mol, 而C=C键的键能为610 kJ/mol,光谱和电 子衍射测定证明乙烯的平面结构如下:

H 121.7°..H.1116.60CC0.110nm0.133nmHH
o o 0.110nm 121.7 116.6 0.133nm C C H H H H
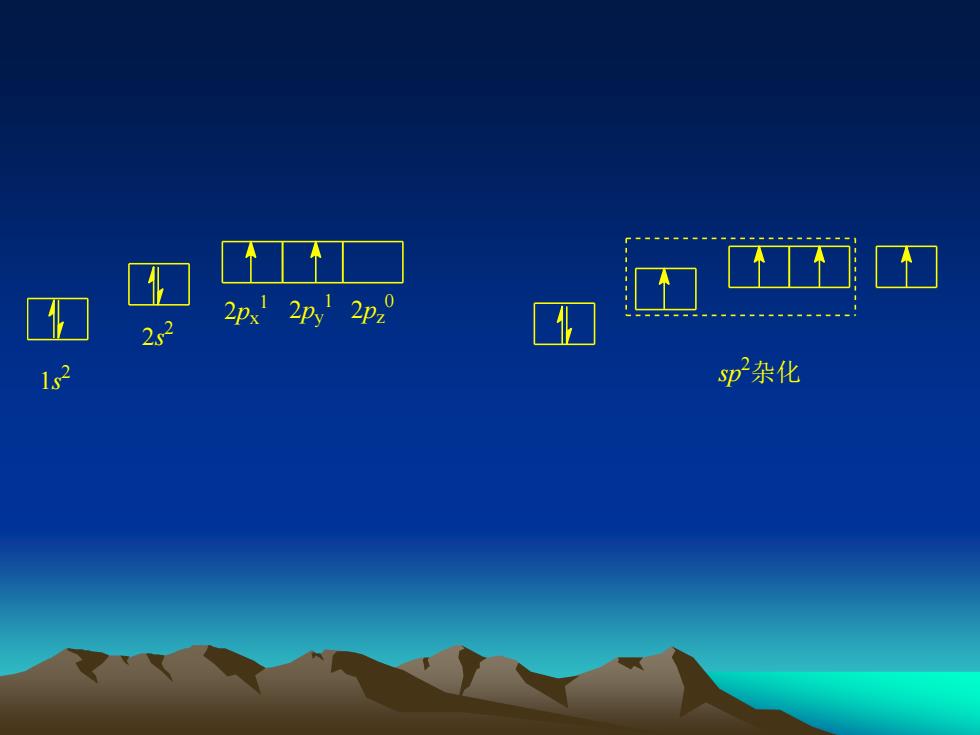
2px2py2p22ssp杂化1s
1s 2 2s 2 2px 1 2py 1 2pz 0 sp 2杂化
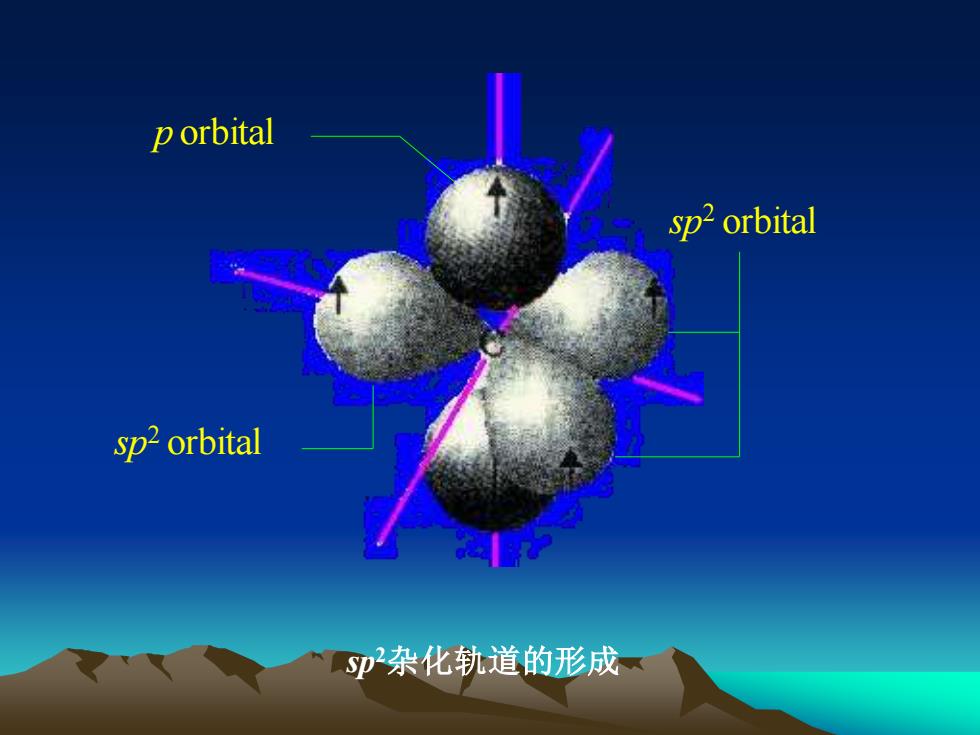
p orbitalsp? orbitalsp?orbitalsp杂化轨道的形成
sp2杂化轨道的形成 sp2 orbital sp2 orbital p orbital
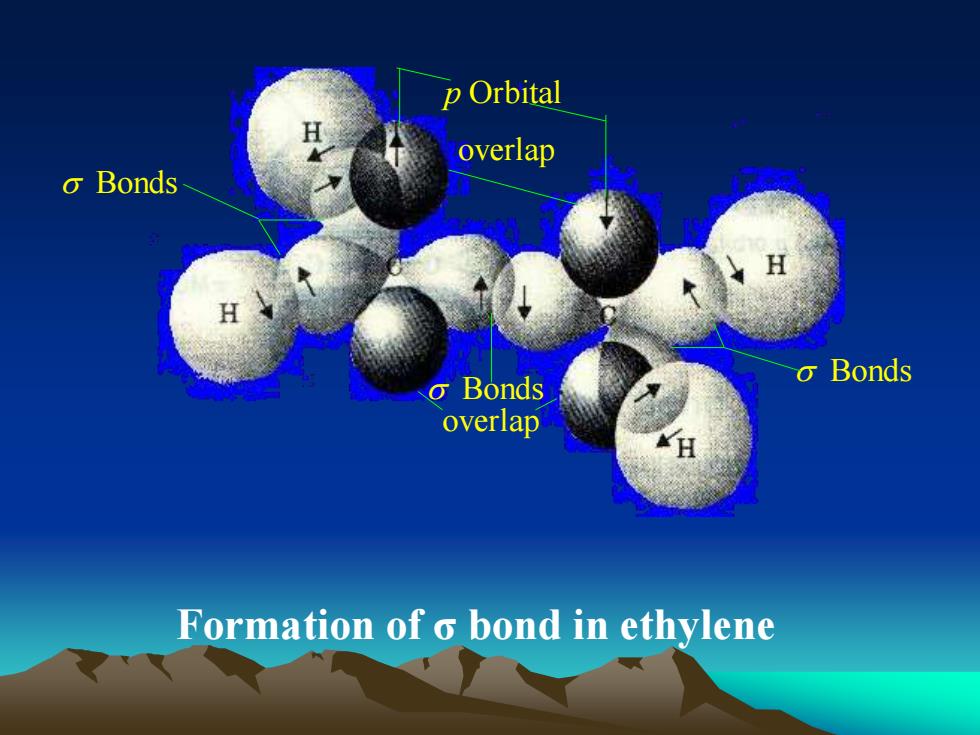
p OrbitalHoverlapo BondsHgBondsoBondsoverlapHFormation of bond in ethylene
Bonds Bonds Bonds overlap p Orbital overlap Formation of σ bond in ethylene
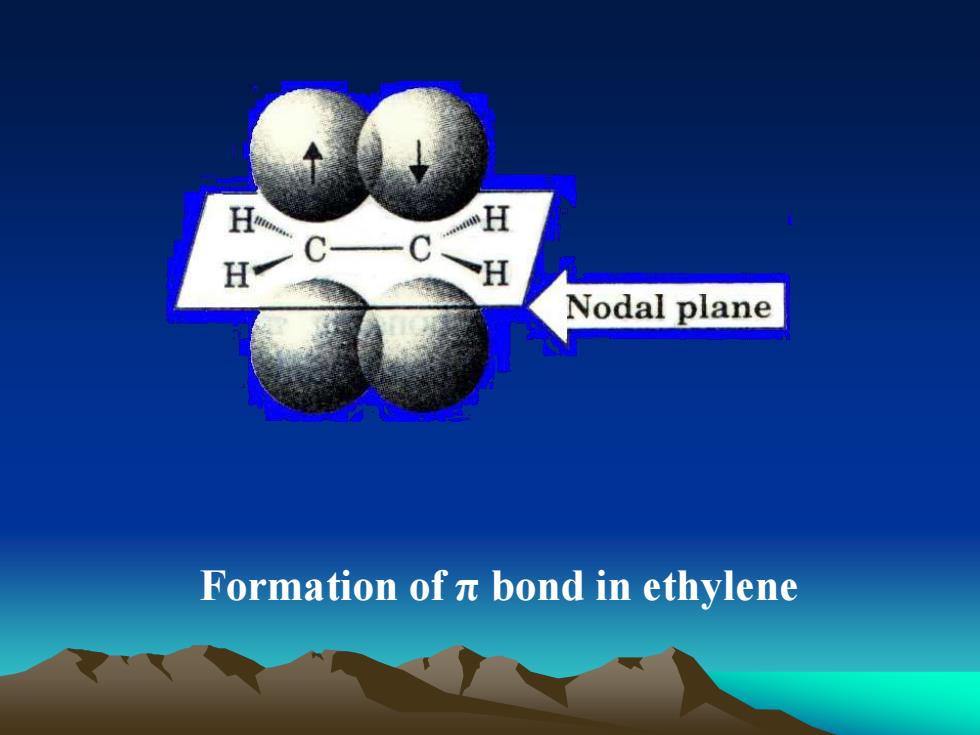
HHHHNodalplaneFormation of 元 bondinethylene
Formation of π bond in ethylene
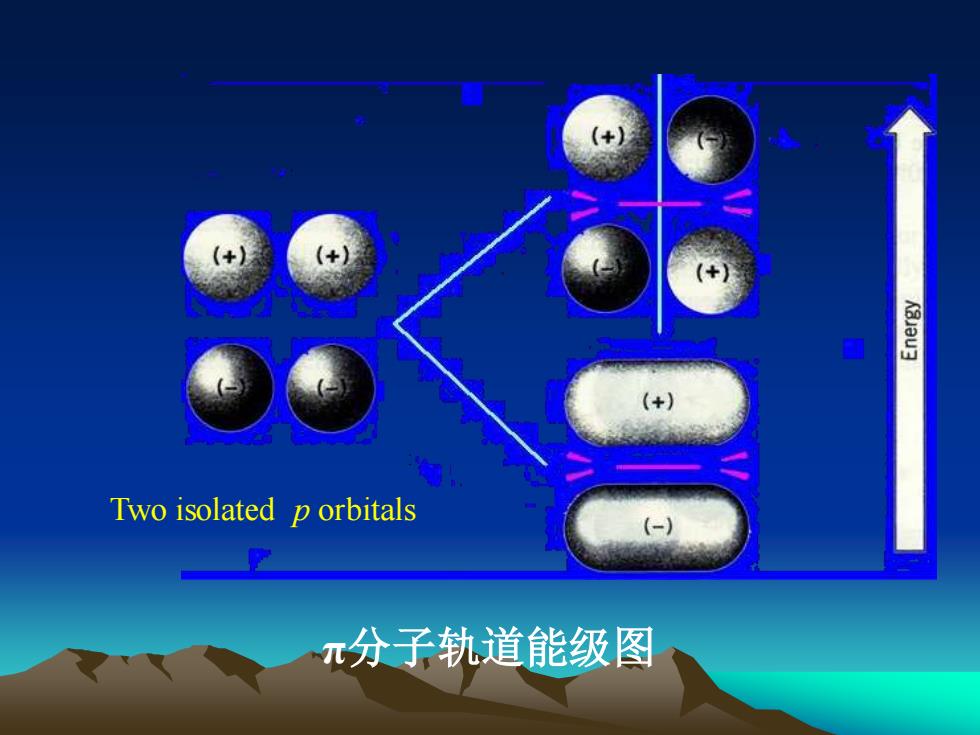
ieTwo isolated p orbitals-元分子轨道能级图
Two isolated p orbitals π分子轨道能级图
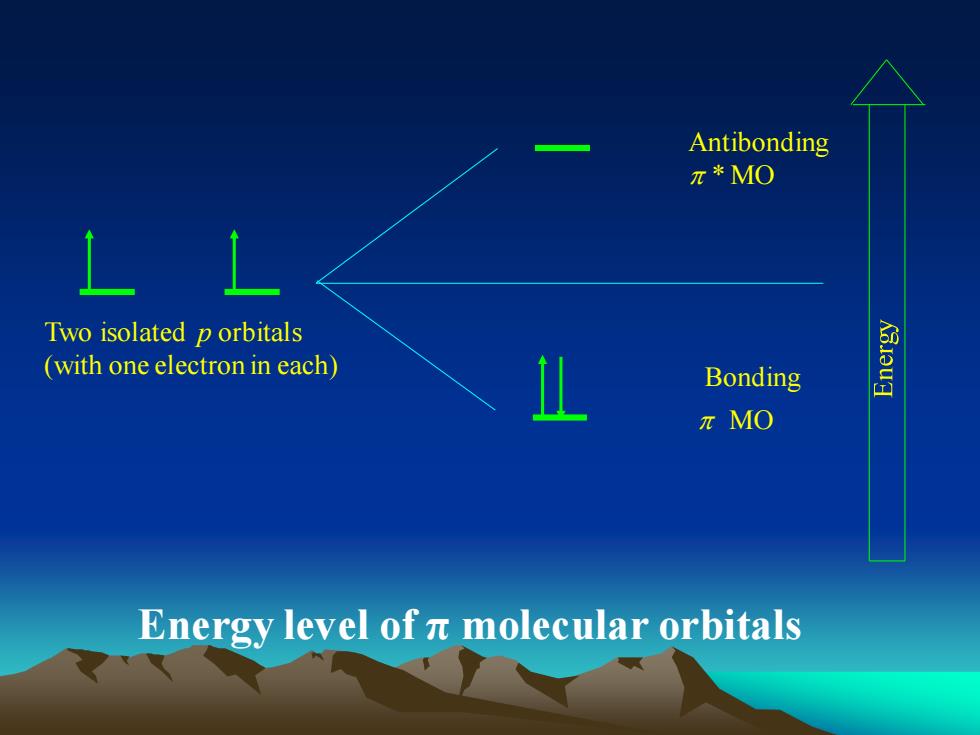
Antibonding元*MO1Two isolated porbitals(withoneelectronineach)BondingI元MOEnergy level of π molecular orbitals
Antibonding * MO Bonding MO Two isolated p orbitals (with one electron in each) Energy level of π molecular orbitals