
Chapter 4.Alkynes and Dienes
Chapter 4. Alkynes and Dienes

1 Alkynes4.1 Structure ofAcetyleneporbitalssp hybrid orbitalsp hybrid orbital
1 Alkynes 4.1 Structure of Acetylene p orbitals sp hybrid orbital sp hybrid orbital
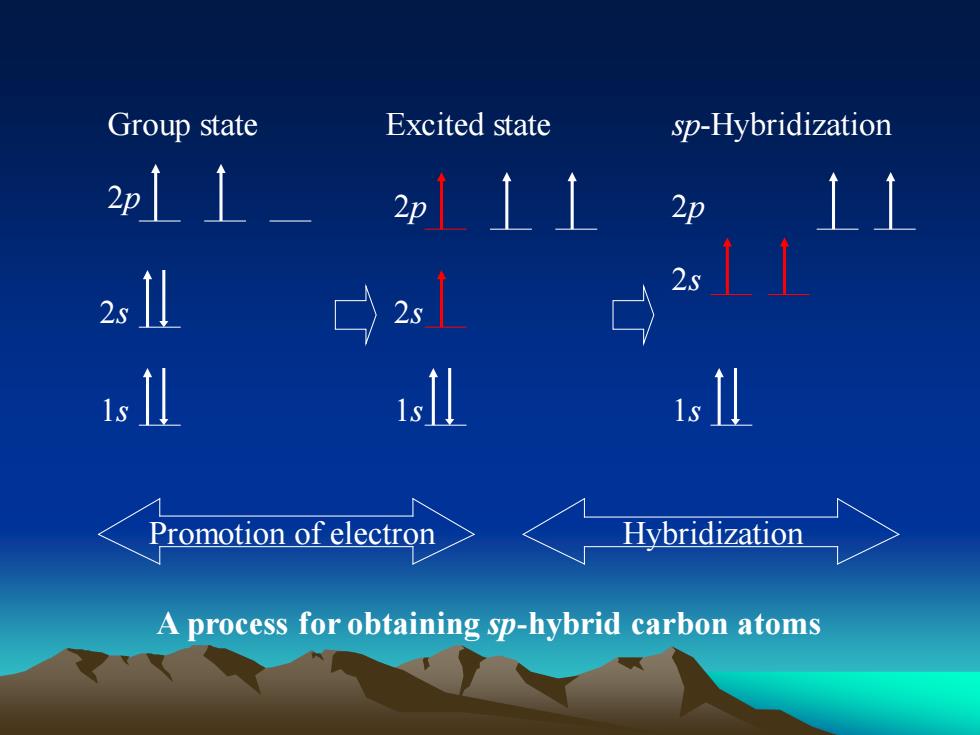
Group stateExcitedstatesp-Hybridization2p/2p2p2s↑2s1s Il11s1sPromotionofelectronHybridizationAprocess forobtaining sp-hybrid carbon atoms
Promotion of electron A process for obtaining sp-hybrid carbon atoms Hybridization 2p 2s 1s Group state 1s 2p 2s Excited state 2p 2s 1s sp-Hybridization
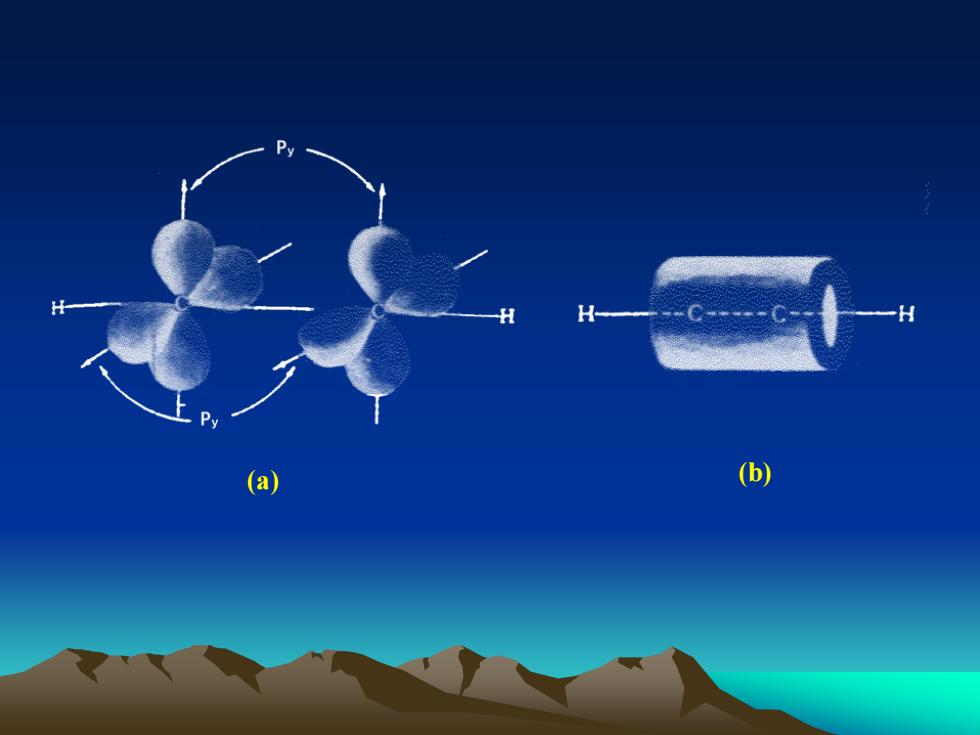
HH(b)a
(a) (b)
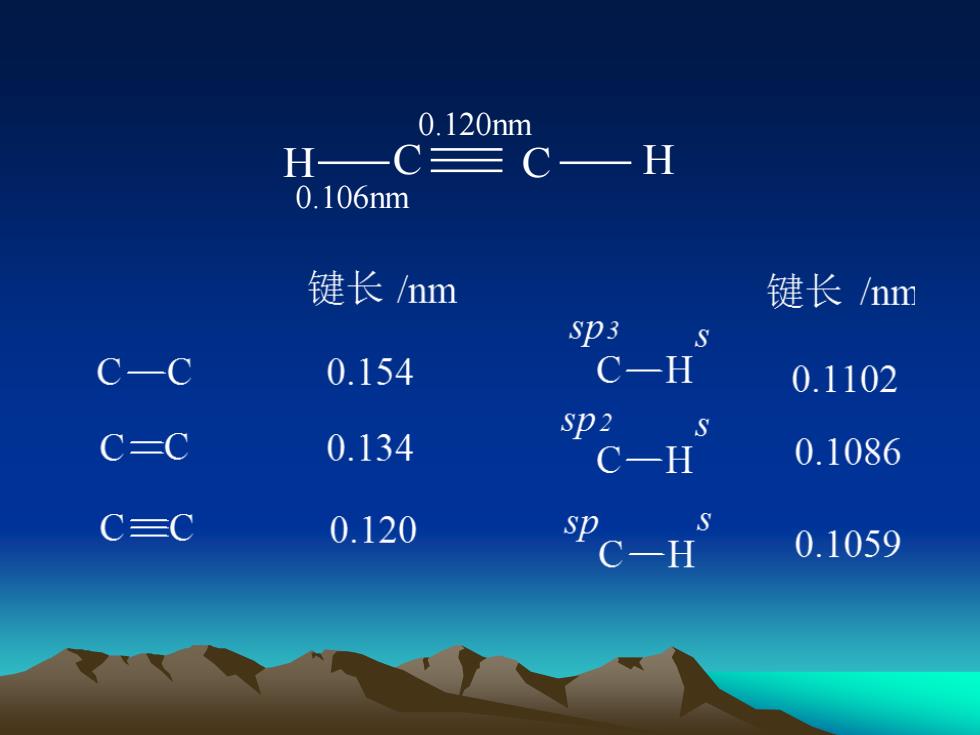
0.120nmH-CH-0.106nm键长/nm键长/nmsp3SC-HC-C0.1540.1102sp2SC=C0.1340.1086C-HSCCsp0.1200.1059C-H
H C C H 0.120nm 0.106nm
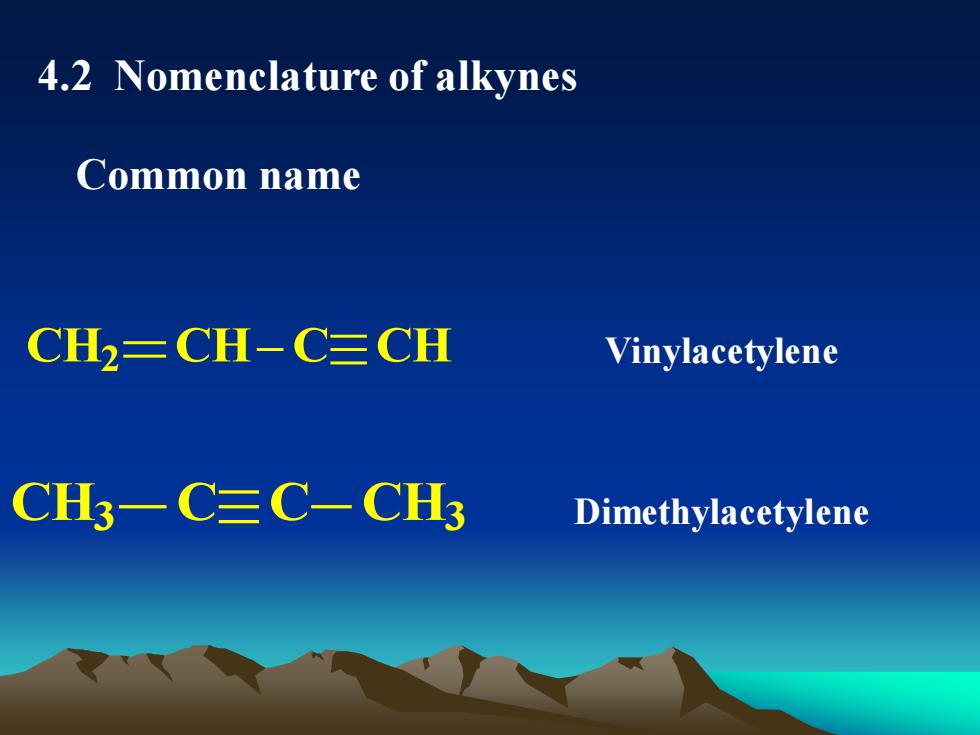
4.2 Nomenclature ofalkynesCommon nameCH2CH-C三CHVinylacetyleneCH3—C三C-CH3Dimethylacetylene
4.2 Nomenclature of alkynes Common name CH2 CH C CH CH3 C C CH3 Vinylacetylene Dimethylacetylene
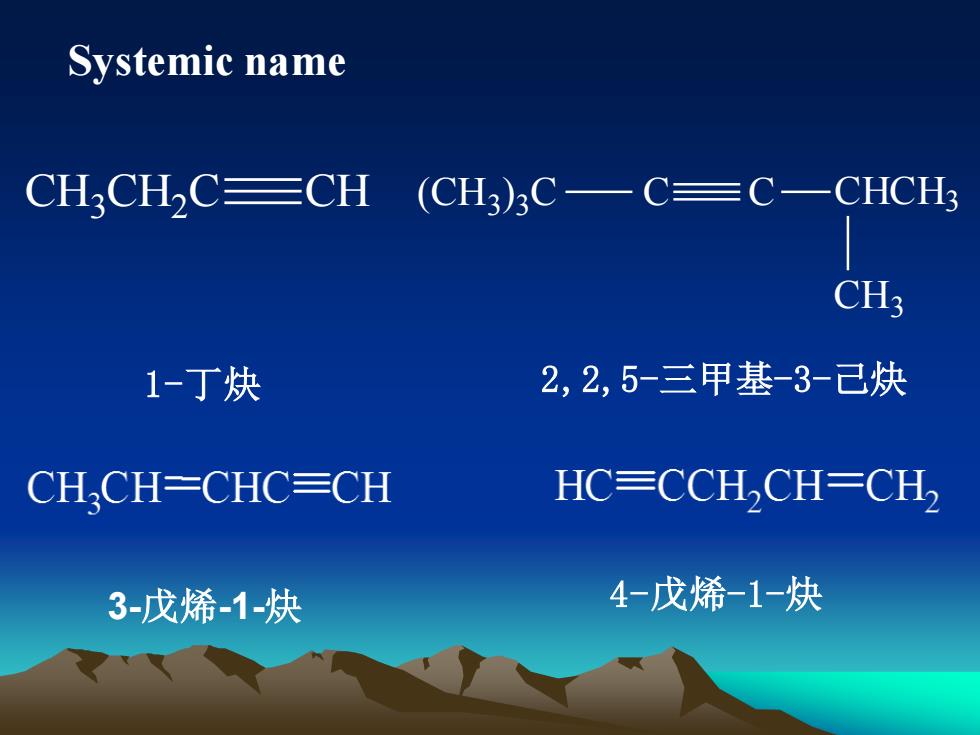
Systemic nameC—CHCH3CHCHC三CH(CH3)3C-CH32,2,5-三甲基-3-已炔1-丁炔HC=CCH,CH=CH,CH.CH=CHC三CH4-戊烯-1-炔3-戊烯-1-炔
Systemic name CH3 CH2 C CH (CH3 ) 3 C C C CHCH3 CH3 1-丁炔 2,2,5-三甲基-3-己炔 3-戊烯-1-炔 4-戊烯-1-炔
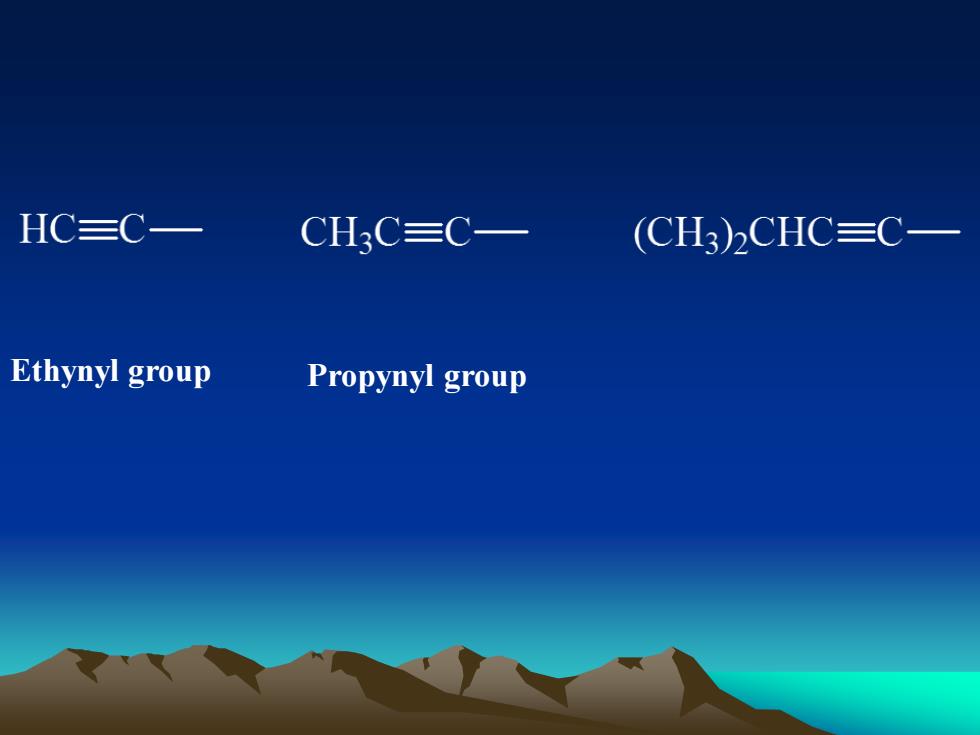
HC三C-CH3C=C—(CH3)2CHC三C-Ethynyl groupPropynyl group
Ethynyl group Propynyl group
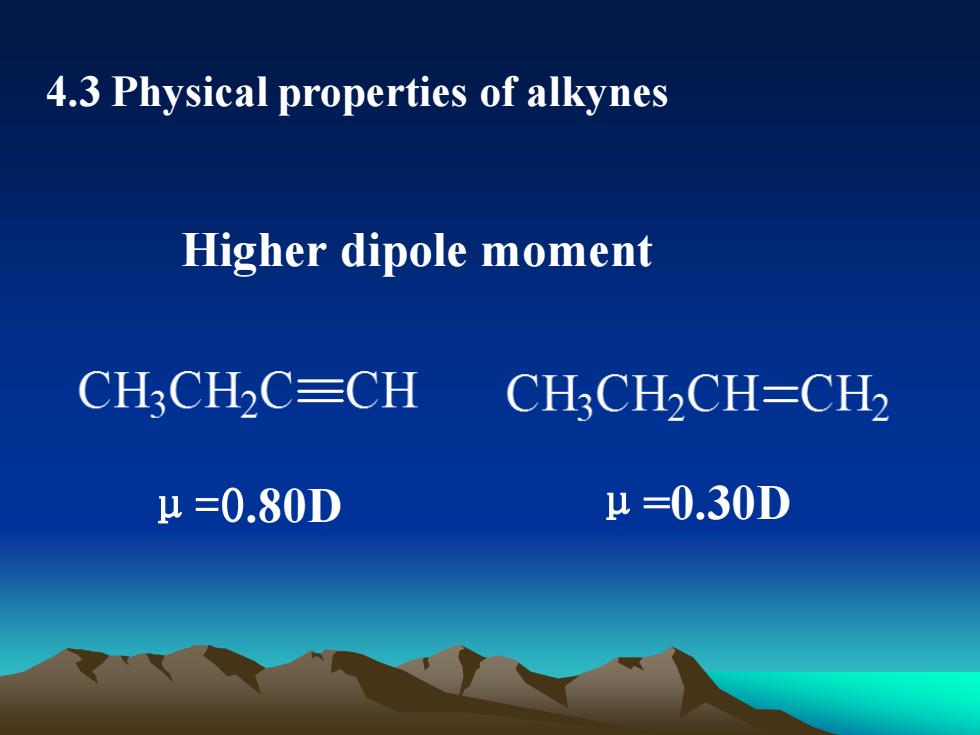
4.3PhysicalpropertiesofalkynesHigherdipolemomentCH,CH,C=CHCH,CHCH=CHμ =0.30DH=0.80D
4.3 Physical properties of alkynes Higher dipole moment μ=0.80D μ=0.30D
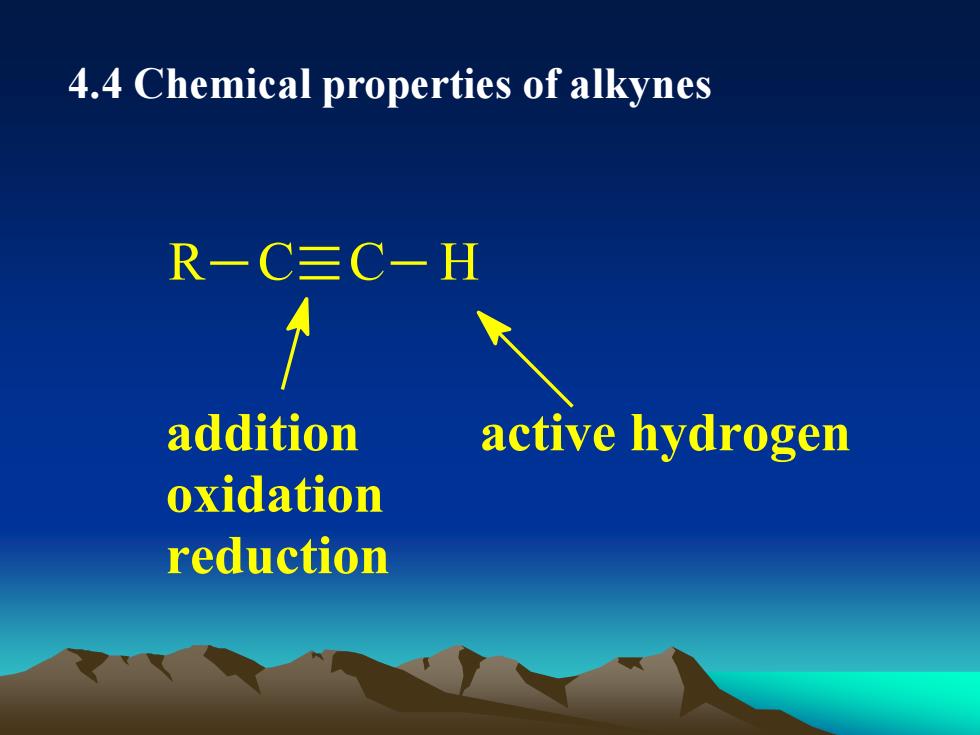
4.4ChemicalpropertiesofalkynesR-C三C-Hadditionactivehydrogenoxidationreduction
4.4 Chemical properties of alkynes R C C H addition oxidation reduction active hydrogen