
Avarlety ofeacioodeavallabt 中一 CHAPTER 9 一扫 Further Reactions of Alcohols and the Chemistry of Ethers 一 一大 9-1 Reactions of Alcohols with Base:Preparation of than that of the alkoxide. 2H-OH 2M(Li.Na.K.C9)-2M'-OH +H CHCH Less Vigorous Alkmnid山,from Athah and Alkai Metasls 2+2二8+ Rclative Rsactivity of ROH with Alkali Mctals R=CH,>primary secondary tertiary Uses for alkoxides 9-2 nes to form alkenes Elimination Reactions of Alcohols halalkaes to ormethers 1
1 CHAPTER 9 Further Reactions of Alcohols and the Chemistry of Ethers A variety of reaction modes are available to alcohols. Reactions of Alcohols with Base: Preparation of Alkoxides 9-1 Strong bases are needed to deprotonate alcohols completely. Base strength must be stronger than that of the alkoxide. Alkali metals also deprotonate alcohols, but by reduction of H+. Vigorous: Less Vigorous: Relative reactivities: Uses for alkoxides: Hindered alkoxides E2 reactions with haloalkanes to form alkenes. Less hindered alkoxides SN2 reactions with haloalkanes to form ethers. Reactions of Alcohols with Strong Acids: Alkyloxonium Ions in Substitution and Elimination Reactions of Alcohols 9-2 Water has a high pKa (15.7) which means that its conjugate base, OH- is an exceedingly poor leaving group. The –OH group of an alcohol must be converted into a better leaving group for alcohols to participate in substitution or elimination reactions

9-2 Reactior of Alcohols th Ste and &Rea8amoa R-H+ R-6 Esroeianrdai6sahna8asggaEa. Come l into 2-Bro m2-mcth 、一6+4aH2◆G、一0+H- temeheeenmCartbeatne2eaemeeagtersceo 品 d (c aretopmary): mpared to tertiary alcohols): Retarded S1 reactivity. Batwtyatahasotmoeamrboeoem 2
2 Reactions of Alcohols with Strong Acids: Alkyloxonium Ions in Substitution and Elimination Reactions of Alcohols 9-2 Haloalkanes from primary alcohols and HX: Water can be a leaving group. Protonation of the hydroxy substituent of an alcohol to form an alkyloxonium ion converts the –OH from the poor leaving group, OH- , to the good leaving group, H2O. Primary bromoalkanes and iodoalkanes can be prepared by the reaction with HBr and HI. Chloroalkanes cannot be prepared by this method because Cl- is too poor a nucleophile. Secondary and tertiary alcohols undergo carbocation reactions with acids: SN1 and E1. Primary alkyloxonium ions undergo only SN2 reactions with acid. Their carbocation transition state energies are too high to allow SN1 and E1 reactions under ordinary laboratory conditions. Secondary and tertiary alkyloxonium ions lose water when treated with acid to form a carbocation. When good nucleophiles are present, the SN1 mechanism predominates. Here the tertiary carbocation is generated at a relatively low temperature, which prevents the competing E1 reaction. At higher temperatures, or in the absence of good nucleophiles, elimination becomes dominant. Secondary alcohols show complex behavior when treated with HX, following SN2, SN1, and E1 pathways. Relatively hindered (compared to primary alcohols): Retarded SN2 reactivity Slow to form carbocations (compared to tertiary alcohols): Retarded SN1 reactivity. E1 reactions of alcohols (dehydrations) result in the formation of alkenes. Non-nucleophilic acids, such as H3PO4 or H2SO4, are used in this case, rather than the nucleophilic acids, HBr and HI. Dehydrations of tertiary alcohols often occur just above room temperature

ents Hydride shifts give n Summary x.RX HRr包,M 3o72a的m 88 eCgoaYacapi9ghepme,edngo arbocation rearrangements also give new E1 aeegg2ceordesoue 3
3 Summary 9-3 Carbocation Rearrangements Hydride shifts give new SN1 products. Treatment of substituted secondary alcohols produces unexpected results: Rearrangement of an initial secondary carbocation to the more stable tertiary carbocation by a hydride shift results in a rearranged product. Hydride shifts are very fast (faster than SN1 or E1) which is partially due to hyperconjugation in the carbocation weakening the C-H bond): Primary carbocations are too unstable to be formed by rearrangement. Secondary or tertiary carbocations equilibrate readily, leading to a mixture of products when trapped by a nucleophile. Carbocation rearrangement takes place regardless of the precursor leading to the carbocation. Carbocation rearrangements also give new E1 products. Under conditions favoring elimination (elevated temperatures and nonnucleophilic media), carbocations can also rearrange to give rearranged products

Shgarbocationreamangementeareduetoalkn &genonrdahec8ieaetsatenle3weaa8creay 然” le can of le Primary alcohols may undergo rearrangement. 9-4 Organic and Inorganic Esters from Alcohols Organic esters are derivatives of carboxylic acids. Inorganicterare theanalou derivatives a2wnw J. CH 9-4 Organic and Inorganic Esters from Alcohols onesresdwithcarboxynicacastoglveorg9nle nic esters ade from alcohols through 的” gro mprcehich beenthe Brmoalkane Synhs by UingPr
4 Other carbocation rearrangements are due to alkyl shifts. Alkyl shifts, rather than hydride shifts, can occur when a carbocation lacks a suitable secondary or tertiary hydrogen next to the positively charged carbon. Alkyl and hydride shifts are faster when leading to a tertiary carbocation than when leading to a secondary carbocation. In the previous haloalkane rearrangement, movement of an ethyl group rather than a hydride would result in a secondary rather than a tertiary carbocation. Exceptions to this general rule can occur as a result of electronic stabilization or steric relief. Primary alcohols may undergo rearrangement. Alkyl and hydride shifts to primary carbons bearing leaving groups can occur without the formation of primary carbocations. In this case, steric hindrance interferes with direct attack by the bromide ion. Instead, water leaves at the same time as the methyl group migrates, bypassing the formation of a primary carbocation. 9-4 Organic and Inorganic Esters from Alcohols Organic esters are derivatives of carboxylic acids. Inorganic esters are the analogous derivatives of inorganic acids. 9-4 Organic and Inorganic Esters from Alcohols Alcohols react with carboxylic acids to give organic esters. Esterification is the reaction of alcohols with carboxylic acids in the presence of catalytic amounts of a strong inorganic acid (H2SO4 or HCl) which yields esters and water. This is an equilibrium process which can be shifted in either direction. Haloalkanes can be made from alcohols through inorganic esters. As an alternative to the acid-catalyzed conversions of alcohols into haloalkanes, a number of inorganic reagents can convert the alcoholic hydroxyl group into a good leaving group under milder conditions. The reaction of PBr3 with a secondary alcohol yields a bromoalkane and phosphorous acid (all three bromine atoms can be utilized)

STEP1 gc品e CHACH-LCH-OH CHCCH HjPOy ASrliuieonatescaiGnYeratlesubstratesfoT 0-S-00 Syudek of an Alyisuknale ”+道总0:一6限·-sg+ 9-5 Names and Physical Properties of Ethers Common Names: CH 5
5 Iodoalkanes can be prepared using PI3, which, because of its reactivity, is generated from red phosphorous and iodine in the reaction mixture itself. Chloroalkanes are commonly prepared using thionyl chloride by warming an alcohol in its presence. An amine such as triethyl amine is usually added to consume the generated HCl. Alkyl sulfonates are versatile substrates for substitution reactions. Alkyl sulfonates are excellent leaving groups and can be generated by the reaction of an alcohol with the corresponding sulfonyl chloride. Pyridine or a tertiary amine is used to remove the HCl formed. Alkyl sulfonates are often crystalline solids and can be isolated and purified before further reaction. The sulfonate group can be displaced by halide ions (particularly in primary and secondary systems). The sulfonate group can also be displaced by any good nucleophile, not just by halide ions as was the case for hydrogen, phosphorous, and thionyl halides. 9-5 Names and Physical Properties of Ethers In the IUPAC system, ethers are alkoxyalkanes. IUPAC: Ethers are alkanes bearing an alkoxy substituent. The larger substituent is the stem and the smaller substituent is the alkoxy group (methoxyethane). Common Names: The names of the two alkyl groups are followed by the word “ether” (ethylmethylether)

unrction Ether Solvents and Their Names anes (tetr 26 Simple alk 33级 e) 10 CH.CHCHOH 824 .CH.CH.CHO crown ethers and methane- completely water soluble 10%aqueous solutior 6
6 Except for strained cyclical derivatives, ethers are fairly unreactive and are often used as solvents in organic reactions. Cyclic ethers are members of the class of cycloalkanes called heterocycles, in which one or more carbon atoms have been replaced by a heteroatom. Cyclic ethers’ names are based on the oxacycloalkane stem: Oxacyclopropane (oxiranes, epoxides, ethylene oxides) Oxacyclobutane Oxacyclopentane (tetrahydrofurans) Oxacyclohexanes (tetrahydropyrans) Ring numbering starts on the oxygen atom. Cyclic polyethers based on the 1,2-ethanediol unit are called crown ethers. The crown ether 18-crown-6 contains 18 total atoms and 6 oxygen atoms. Note that the inside of the ring is electron rich. The absence of hydrogen bonding affects the physical properties of ethers. Simple alkoxyalkanes have the same molecular formula as the equivalent alkanols, CnH2n+2O, but have much lower boiling points due to the absence of hydrogen bonding. The smaller alkoxyalkanes are water soluble, however solubility decreases with increasing hydrocarbon size. Methoxymethane — completely water soluble Ethoxyethane — 10% aqueous solution Polyethers solvate metal ions: crown ethers and ionophores. Crown ethers can render salts soluble in organic solvents by chelating the metal cations. This allows reagents such as KMnO4 to act as an oxidizing agent in the organic solvents. The size of the central cavity can be tailored to selectively bind cations of differing ionic radii

5heVSaewpe%oooenfanowat0h o- by intramolecular -0 mall rings can be prepared using the eae.otrngchsuresbasedonbothenhalpcandent Relative Rates of Cyelie Ether Formation 7
7 Three-dimensional analogs of crown ethers are polyethers called cryptands. These are highly selective in alkali and other metal cation binding. 9-6 Williamson Ether Synthesis Ethers are prepared by SN2 reactions. Ethers can be prepared by the reaction of an alkoxide with a primary haloalkane or sulfonate ester under SN2 conditions. The parent alcohol of the alkoxide can be used as the solvent, however other polar solvents are often better, such as DMSO (dimethyl sulfoxide) or HMPA (hexamethylphosphoric triamide). The use of alkoxides in ether synthesis is restricted to primary unhindered alkylating agents, otherwise significant amounts of E2 products are formed. Cyclic ethers can be prepared by intramolecular Williamson synthesis. Haloalcohols serve as the starting point for the Williamson synthesis of cyclic ethers. The intramolecular reaction is usually much faster than the intermolecular reaction. If necessary, the intermolecular reaction can be suppressed by using a high dilution of the haloalcohol. Cyclic ethers of even small rings can be prepared using the Williamson synthesis. Ring size controls the speed of cyclic ether formation. The rate of ring closure is based on both enthalpic and entropic contributions. These rate differences can be explained based on the interplay between strain, entropy, and proximity. Entropy reduction (due to ring closure) increases with increasing ring size. (Reaction rate decrease with increasing ring size.) Ring strain decreases with increasing ring size. (Reaction rate increase with increasing ring size.) Transition-state strain is reduced in the 2-haloalkoxides because the 2-haloalkoxide is already strained by the proximity of the halide and hydroxyl. (Reaction rate increase for the 2-haloalkoxides.)

Wlamson synthesls is 9-7 Synthesis of Ethers:Alcohols and Mineral Acid 品80 2weCaasHB,Hyedhaloakanswhen Teea9eRmbeonhae6eeothene6e地 c acids vields ethers when reacted wit 2M CHOMCONO h含andas sf ther4 thes eeeepdng,smeionofwatarocaurw Q101-0 also forn 9-8 Reactions of Ethers 之品 二学 ++ 8
8 The intramolecular Williamson synthesis is stereospecific. Since the Williamson synthesis is a SN2 substitution reaction, an inversion of configuration occurs at the carbon bearing the leaving group. The leaving group must be on the opposite side of the molecule from the attacking nucleophile in order for the reaction to occur. 9-7 Synthesis of Ethers: Alcohols and Mineral Acid Alcohols give ethers by both SN2 and SN1 mechanisms. Strong nucleophilic acids (HBr, HI) yield haloalkanes when reacted with alcohols. Strong non-nucleophilic acids yields ethers when reacted with alcohols. At higher temperatures, an E2 elimination of water occurs with the subsequent production of alkenes. Secondary and tertiary alcohols form ethers through an SN1 reaction with a second molecule of the alcohol trapping the carbocation. The E1 pathway becomes dominate at higher temperatures. Mixed ethers containing one tertiary and one primary or secondary alcohol can be prepared in the presence of dilute acid. The tertiary carbocation is trapped by the less hindered alcohol. Ethers also form by alcoholysis. This occurs by simply dissolving a tertiary or secondary haloalkane in an alcohol and waiting until the SN1 process is complete. 9-8 Reactions of Ethers Ethers are usually inert, however, they do react slowly with oxygen to form hydroperoxides and peroxides which can decompose explosively

9-8 Reactions of Ethers etheroy9enatomcanbeprotonatedogenerate C38cac2Yeaanomtystes2 gp2em2n9aue5nhasognudeophcacsHB,s2 :OH CHRUOUOG CC ertiary ethers are protecting groups for alc ols. 9-9 Reactions of Oxacyclopropanes be. a+a号aa The driving force for this reaction is the release of ring strain Carbon8emeam6y8ec,Bcteee8ew8aeew ert strained ether cyopropestovieldacohos 几 9
9 9-8 Reactions of Ethers The ether oxygen atom can be protonated to generate alkyloxonium ions. With primary groups and strong nucleophilic acids (HBr), SN2 displacement takes place. Oxonium ions from secondary ethers may transform by either SN2 or SN1 reactions, depending upon conditions. Tertiary ethers are protecting groups for alcohols. Esters containing tertiary alkyl groups react in dilute acid to give carbocations which are either trapped (SN1) by good nucleophiles or deprotonated in the absence of good nucleophiles. Because they are readily formed (and equally readily hydrolyzed), tertiary ethers are commonly used as protecting groups during chemical reactions which might otherwise interact with the unprotected alcohol. 9-9 Reactions of Oxacyclopropanes Nucleophilic ring opening of oxacyclopropanes by SN2 is regioselective and stereospecific. Oxacyclopropane can be ring-opened by anionic nucleophiles. Because the molecule is symmetric, nucleophilic attack can be at either carbon atom. The driving force for this reaction is the release of ring strain. With unsymmetric systems, attack will be at the less substituted carbon center. This selectivity is referred to as “regioselectivity.” If the ring opens at a stereocenter, inversion is observed. Hydride and organometallic reagents convert strained ethers into alcohols. LiAlH4 can open the rings of oxacyclopropanes to yield alcohols. (Ordinary ethers do not react.) In asymmetrical systems, the hydride attacks the less substituted side
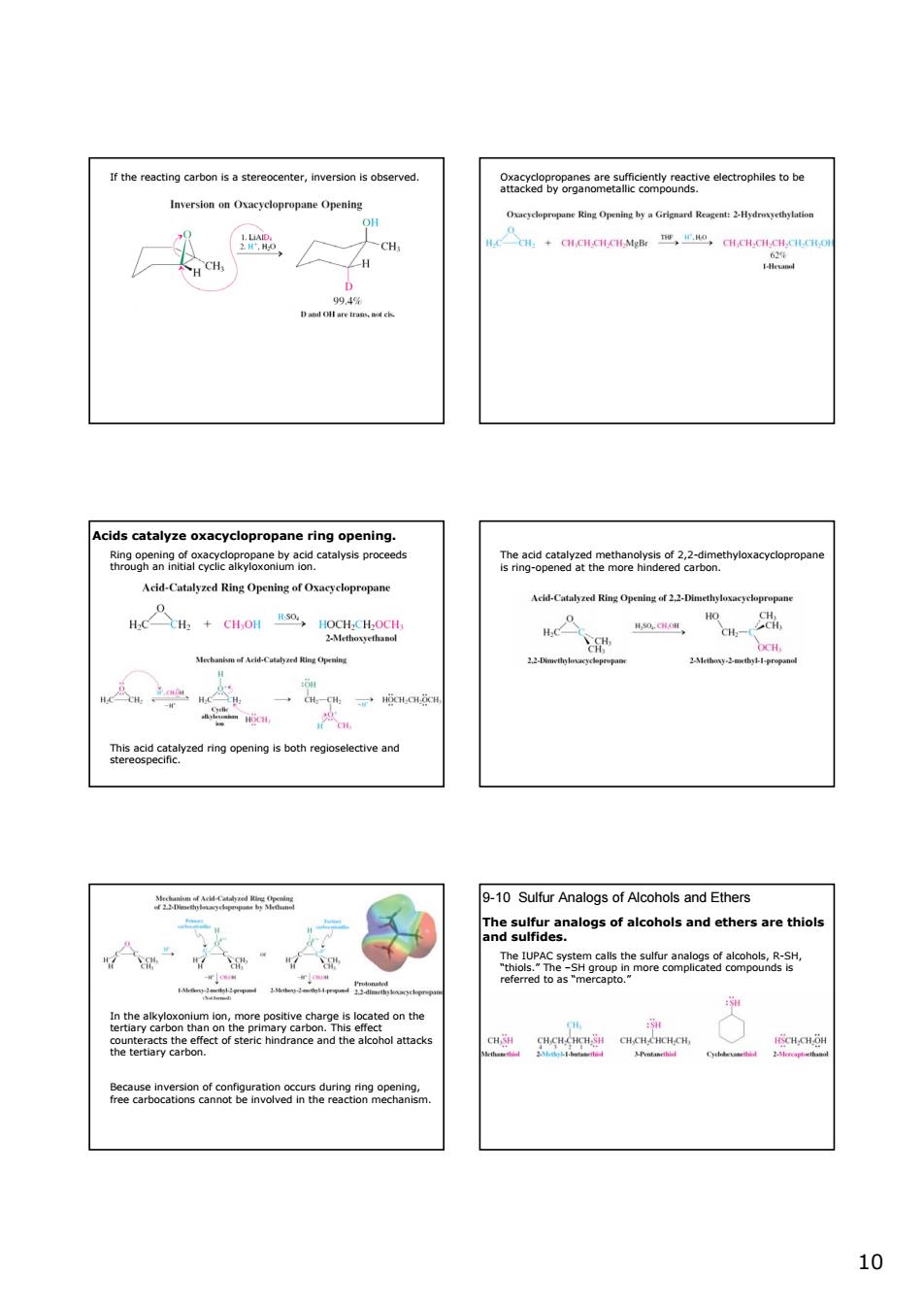
r therciner,inversion served. 9nacxeg8weaeiomeaeeamgoeseuecrophlsotbe -CH Acids catalyze oxacyclopr onane ring on eds ggataaeta0k.2rtahyhoxaqycopropan Acd-Catalyzed Ring Opening ofxayclopropane cd-Cal Ring Opmleg f Hc+CH.OH,a4C 0. 9-10 Sulfur Analogs of Alcohols and Ethers hgumeanalogsofalcoholsandethersarethiols 10
10 If the reacting carbon is a stereocenter, inversion is observed. Oxacyclopropanes are sufficiently reactive electrophiles to be attacked by organometallic compounds. Acids catalyze oxacyclopropane ring opening. Ring opening of oxacyclopropane by acid catalysis proceeds through an initial cyclic alkyloxonium ion. This acid catalyzed ring opening is both regioselective and stereospecific. The acid catalyzed methanolysis of 2,2-dimethyloxacyclopropane is ring-opened at the more hindered carbon. In the alkyloxonium ion, more positive charge is located on the tertiary carbon than on the primary carbon. This effect counteracts the effect of steric hindrance and the alcohol attacks the tertiary carbon. Because inversion of configuration occurs during ring opening, free carbocations cannot be involved in the reaction mechanism. 9-10 Sulfur Analogs of Alcohols and Ethers The sulfur analogs of alcohols and ethers are thiols and sulfides. The IUPAC system calls the sulfur analogs of alcohols, R-SH, “thiols.” The –SH group in more complicated compounds is referred to as “mercapto