
Lecture 23 Transgenes and Gene Targeting in Mice I In the next two lectures I will be telling you about some of the ways in which we can study gene function in higher eukaryotes,more specifically in the laboratory mouse Mus Musculus.I will be doing this by telling you about a remarkable number of manipulations that have been made to the mouse genome in order to generate an experimental mouse model system for human Sickle Cell Disease.The mouse that was developed to explore this human disease turns out to be one of most genetically modified mice on the planet...and so it gives us an interesting framework in which to tell you about making transgenic and knockout mice.To set the scene for genetically modifying mice to mimic human sickle cell disease we need to step back a bit and consider this devastating human disease and some of its features. Human Sickle Cell Disease (a.k.a.sickle cell anemia):Sickle cell disease is a human blood disorder that is caused by a single mutation in a gene that encodes one of the subunits of hemoglobin(Hb),namely B-globin. Sickle Cell Disease-An autosomal Hemoglobin is a tetrameric protein made up Recessive disorder of Hemoglobin of two a-globin proteins,and two B-globin proteins;oaββ.Each of the4 globin proteins embrace an iron-containing heme molecule A single mutation in (iron is what makes hemoglobin and Red the sixth amino acid of Blood Cells red)whose function is to bind the B-globin chain (Glutamine oxygen in the lungs and release it in all the Valine) causes Sickle tissues of the animal.The very simple Cell Diseas change of the sixth amino acid in B-globin Images removed due to copyright reasons (glutamine is substituted with a valine) causes devastating consequences.It turns out that Hb containing B-globin subunits with the sickle mutation(known as HbS)does not directly interfere with the ability of hemoglobin to store or release oxygen,but rather this amino acid change bestows a novel property on the hemoglobin molecule;in its deoxygenated state the HbS molecules aggregate together to form polymeric fibers,and the presence of these fibers grossly distort the shape of Red Blood Cells (RBCs).Instead of being shaped almost like a doughnut (without the actual hole)and having tremendous flexibility to squeeze through tiny Images removed due to copyright reasons. capillaries within tissues,the aggregated HbS fibers cause the RBCs to become curved(like a sickle),rigid,prone to rupture and prone to clumping;rupture causes anemia and clumping clogs small blood vessels, leading to tissue damage
Lecture 23 Transgenes and Gene Targeting in Mice I In the next two lectures I will be telling you about some of the ways in which we can study gene function in higher eukaryotes, more specifically in the laboratory mouse Mus Musculus. I will be doing this by telling you about a remarkable number of manipulations that have been made to the mouse genome in order to generate an experimental mouse model system for human Sickle Cell Disease. The mouse that was developed to explore this human disease turns out to be one of most genetically modified mice on the planet…and so it gives us an interesting framework in which to tell you about making transgenic and knockout mice. To set the scene for genetically modifying mice to mimic human sickle cell disease we need to step back a bit and consider this devastating human disease and some of its features. Human Sickle Cell Disease (a.k.a. sickle cell anemia): Sickle cell disease is a human blood disorder that is caused by a single mutation in a gene that encodes one of the subunits of hemoglobin (Hb), namely β-globin. Hemoglobin is a tetrameric protein made up of two α-globin proteins, and two β-globin proteins; ααββ. Each of the 4 globin proteins embrace an iron-containing heme molecule (iron is what makes hemoglobin and Red Blood Cells red) whose function is to bind oxygen in the lungs and release it in all the tissues of the animal. The very simple change of the sixth amino acid in β-globin (glutamine is substituted with a valine) causes devastating consequences. It turns out that Hb containing β-globin subunits with the sickle mutation (known as HbS) does not directly interfere with the ability of hemoglobin to store or release oxygen, but rather this amino acid change bestows a novel property on the hemoglobin molecule; in its deoxygenated state the HbS molecules aggregate together to form polymeric fibers, and the presence of these fibers grossly distort the shape of Red Blood Cells (RBCs). Instead of being shaped almost like a doughnut (without the actual hole) and having tremendous flexibility to squeeze through tiny capillaries within tissues, the aggregated HbS fibers cause the RBCs to become curved (like a sickle), rigid, prone to rupture and prone to clumping; rupture causes anemia and clumping clogs small blood vessels, leading to tissue damage. Sickle Cell Disease – An autosomal Recessive disorder of Hemoglobin A single mutation in the sixth amino acid of the β-globin chain (Glutamine -> Valine) causes Sickle Cell Disease • Red blood cells (RBCs) make up 40% of the blood volume • Hemoglobin makes up 70% of the proteins in RBCs Sickle Cell Disease – An autosomal Recessive disorder of Hemoglobin A single mutation in the sixth amino acid of the β-globin chain (Glutamine -> Valine) causes Sickle Cell Disease • Red blood cells (RBCs) make up 40% of the blood volume • Hemoglobin makes up 70% of the proteins in RBCs Images removed due to copyright reasons. Images removed due to copyright reasons
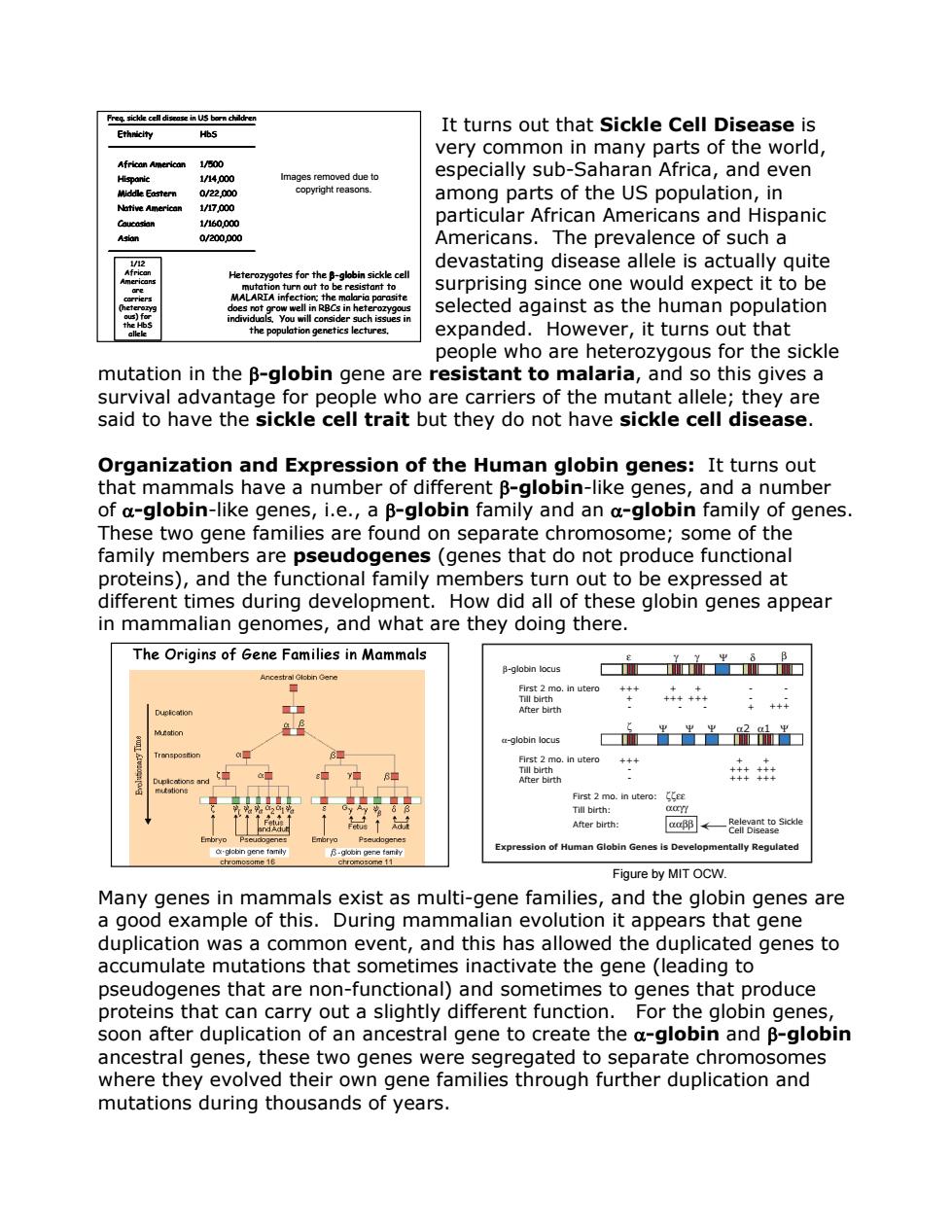
Freg.sickle cell disease in US born children Ethnicity It turns out that Sickle Cell Disease is HbS very common in many parts of the world, African Americon 1/B00 Hispanic 1/14.000 Images removed due to especially sub-Saharan Africa,and even Middle Eastern 0/22.000 copyright reasons. among parts of the US population,in Native American 1/17,000 1/160,000 particular African Americans and Hispanic Asian 0/200,000 Americans.The prevalence of such a 112 devastating disease allele is actually quite African Heterozygotes for the gbin sickle cell wRat2Cot书2 prie surprising since one would expect it to be does not grow well in RBCs in heterozygous selected against as the human population individuals.You will consider such issues in the population genetics lectures. expanded.However,it turns out that people who are heterozygous for the sickle mutation in the B-globin gene are resistant to malaria,and so this gives a survival advantage for people who are carriers of the mutant allele;they are said to have the sickle cell trait but they do not have sickle cell disease. Organization and Expression of the Human globin genes:It turns out that mammals have a number of different B-globin-like genes,and a number of a-globin-like genes,i.e.,a B-globin family and an a-globin family of genes These two gene families are found on separate chromosome;some of the family members are pseudogenes (genes that do not produce functional proteins),and the functional family members turn out to be expressed at different times during development.How did all of these globin genes appear in mammalian genomes,and what are they doing there. The Origins of Gene Families in Mammals E yΨ 8 B-globin locus i加Ti▣ Ancestral Globin Gene First 2 mo.in utero +++ Till birth ++++++ After birth + a-globin locus 出出出品品出 First 2 mo.in utero +++ Till birth Duplcations and After birth 年出 mustions First 2 mo.in utero:E Till birth: After birth: aapp← elckle ne clobin gene family B-globn gene tamiy Expression of Human Globin Genes is Developmentally Regulated 5e11 Figure by MIT OCW. Many genes in mammals exist as multi-gene families,and the globin genes are a good example of this.During mammalian evolution it appears that gene duplication was a common event,and this has allowed the duplicated genes to accumulate mutations that sometimes inactivate the gene(leading to pseudogenes that are non-functional)and sometimes to genes that produce proteins that can carry out a slightly different function.For the globin genes, soon after duplication of an ancestral gene to create the a-globin and B-globin ancestral genes,these two genes were segregated to separate chromosomes where they evolved their own gene families through further duplication and mutations during thousands of years
It turns out that Sickle Cell Disease is very common in many parts of the world, especially sub-Saharan Africa, and even among parts of the US population, in particular African Americans and Hispanic Americans. The prevalence of such a devastating disease allele is actually quite surprising since one would expect it to be selected against as the human population expanded. However, it turns out that people who are heterozygous for the sickle mutation in the β-globin gene are resistant to malaria, and so this gives a survival advantage for people who are carriers of the mutant allele; they are said to have the sickle cell trait but they do not have sickle cell disease. Heterozygotes for the β-globin sickle cell mutation turn out to be resistant to MALARIA infection; the malaria parasite does not grow well in RBCs in heterozygous individuals. You will consider such issues in the population genetics lectures. Ethnicity African American Hispanic Middle Eastern Native American Caucasian Asian HbS 1/500 1/14,000 0/22,000 1/17,000 1/160,000 0/200,000 Freq. sickle cell disease in US born children 1/12 African Americans are carriers (heterozyg ous) for the HbS allele Heterozygotes for the β-globin sickle cell mutation turn out to be resistant to MALARIA infection; the malaria parasite does not grow well in RBCs in heterozygous individuals. You will consider such issues in the population genetics lectures. Ethnicity African American Hispanic Middle Eastern Native American Caucasian Asian HbS 1/500 1/14,000 0/22,000 1/17,000 1/160,000 0/200,000 Freq. sickle cell disease in US born children Ethnicity African American Hispanic Middle Eastern Native American Caucasian Asian HbS 1/500 1/14,000 0/22,000 1/17,000 1/160,000 0/200,000 Ethnicity African American Hispanic Middle Eastern Native American Caucasian Asian HbS 1/500 1/14,000 0/22,000 1/17,000 1/160,000 0/200,000 Freq. sickle cell disease in US born children 1/12 African Americans are carriers (heterozyg ous) for the HbS allele Organization and Expression of the Human globin genes: It turns out that mammals have a number of different β-globin-like genes, and a number of α-globin-like genes, i.e., a β-globin family and an α-globin family of genes. These two gene families are found on separate chromosome; some of the family members are pseudogenes (genes that do not produce functional proteins), and the functional family members turn out to be expressed at different times during development. How did all of these globin genes appear in mammalian genomes, and what are they doing there. The Origins of Gene Families in Mammals Many genes in mammals exist as multi-gene families, and the globin genes are a good example of this. During mammalian evolution it appears that gene duplication was a common event, and this has allowed the duplicated genes to accumulate mutations that sometimes inactivate the gene (leading to pseudogenes that are non-functional) and sometimes to genes that produce proteins that can carry out a slightly different function. For the globin genes, soon after duplication of an ancestral gene to create the α-globin and β-globin ancestral genes, these two genes were segregated to separate chromosomes where they evolved their own gene families through further duplication and mutations during thousands of years. Expression of Human Globin Genes is Developmentally Regulated ε γγ Ψ δ β ζ ΨΨΨ α2 Ψ α-globin locus β-globin locus α1 Relevant to Sickle Cell Disease First 2 mo. in utero: ζζεε Till birth: ααγγ After birth: ααββ First 2 mo. in utero Till birth After birth +++ - - + +++ +++ + +++ +++ First 2 mo. in utero Till birth After birth +++ + - + +++ - - - + - - +++ + +++ - Figure by MIT OCW. Images removed due to copyright reasons

Healthy people:aaBB It is the aaBsBs hemoglobin molecule expressed Images removed due after birth that is responsible for aggregating Sickle Cell Trait:aaBBs to copyright reasons and causing sickle cell disease.The aaBBs hemoglobin tetramers expressed in people heterozygous for the sickle mutation do not Sickle Cell Disease:BBs aggregate to form fibers,and so do not cause disease;however,should such heterozygous aaBsBs is soluble when oxygenated,but precipitates in low oxygen people live at high altitude some sickling can occur. It is sobering to note that almost 50 years since the molecular basis of this disease was discovered there still does not exist a really effective therapy for the disease.Hemoglobin was one of first proteins to be purified,it's gene was one of the first to be cloned,and the globin proteins were among the first to have their structure determined by x-ray crystallography...and although some progress has been made in therapy,much more still needs to be done.This is precisely why having a robust mouse model for sickle cell disease to test experimental therapies is absolutely critical.Tremendous strides have been made in generating a mouse model for sickle cell disease. How do we Genetically modify There are two general ways to specifically the mouse genome? modify the genetic makeup of a mouse.One involves the random integration of a cloned (1)Transgenes gene somewhere into the mouse genome(i.e., adding genes by pronuclear injection the introduction of a "transgene").The other random insertion with no replacement involves precisely targeting a specific gene in (2)"Knock-outs" the mouse and introducing a know alteration of subtracting or deleting genes that gene,usually the deletion of the gene and gene fargeting specific insertion with replacement the insertion of a marker gene in its place (a gene knock-out by targeted homologous recombination). Introduction of the Human B-globin gene with the sickle cell mutation (BsM)into the mouse genome:In the 1980's and early 1990's several groups tried to make a mouse with sickle cell disease by introducing the Human B-globin gene with the sickle mutation(BsM),in the hope that if the protein was expressed at high levels it would precipitate Hb fibers that would cause sickling of RBCs,thus mimicking sickle cell disease.How does one make a transgenic mouse? Mice are treated with a hormone to make them super-ovulate and then mated. Soon after mating,the fertilized eggs are retrieved from the uterus.Eggs that contain two pronuclei(one from the mother and one from the father)indicating that the embryo is still at the one-cell stage,are identified under the
It is the ααβSβS hemoglobin molecule expressed after birth that is responsible for aggregating and causing sickle cell disease. The ααββS hemoglobin tetramers expressed in people heterozygous for the sickle mutation do not aggregate to form fibers, and so do not cause disease; however, should such heterozygous people live at high altitude some sickling can occur. Healthy people: ααββ Sickle Cell Trait: ααββs Sickle Cell Disease: ααβsβs ααβsβs is soluble when oxygenated, but precipitates in low oxygen Healthy people: ααββ Sickle Cell Trait: ααββs Sickle Cell Disease: ααβsβs ααβsβs is soluble when oxygenated, but precipitates in low oxygen It is sobering to note that almost 50 years since the molecular basis of this disease was discovered there still does not exist a really effective therapy for the disease. Hemoglobin was one of first proteins to be purified, it’s gene was one of the first to be cloned, and the globin proteins were among the first to have their structure determined by x-ray crystallography…and although some progress has been made in therapy, much more still needs to be done. This is precisely why having a robust mouse model for sickle cell disease to test experimental therapies is absolutely critical. Tremendous strides have been made in generating a mouse model for sickle cell disease. There are two general ways to specifically modify the genetic makeup of a mouse. One involves the random integration of a cloned gene somewhere into the mouse genome (i.e., the introduction of a “transgene”). The other involves precisely targeting a specific gene in the mouse and introducing a know alteration of that gene, usually the deletion of the gene and the insertion of a marker gene in its place (a gene knock-out by targeted homologous recombination). How do we Genetically modify the mouse genome? (1) Transgenes (2) “Knock-outs” • adding genes by pronuclear injection • random insertion with no replacement • subtracting or deleting genes • gene targeting • specific insertion with replacement How do we Genetically modify the mouse genome? (1) Transgenes (2) “Knock-outs” • adding genes by pronuclear injection • random insertion with no replacement • subtracting or deleting genes • gene targeting • specific insertion with replacement Introduction of the Human β-globin gene with the sickle cell mutation (βS H) into the mouse genome: In the 1980’s and early 1990’s several groups tried to make a mouse with sickle cell disease by introducing the Human β-globin gene with the sickle mutation (βS H), in the hope that if the protein was expressed at high levels it would precipitate Hb fibers that would cause sickling of RBCs, thus mimicking sickle cell disease. How does one make a transgenic mouse? Mice are treated with a hormone to make them super-ovulate and then mated. Soon after mating, the fertilized eggs are retrieved from the uterus. Eggs that contain two pronuclei (one from the mother and one from the father) indicating that the embryo is still at the one-cell stage, are identified under the Images removed due to copyright reasons

microscope.The male pronucleus is injected (still under the microscope)with purified DNA fragments that 4 H mouse. Pronuclel contain the Bs gene along with an appropriate promoter region to STEP 2:Inject cloned human B.H gene (into male pronucleus). Fertiltzed mouse eoa orior give it a good chance of being to fusion of male and female pronuclel expressed in once integrated into STEP 3:Human "transgene" Transfer injected eggs integrates into the mouse into foster mother the genome.The injected DNA genome at a random site. quite often gets incorporated into STEP 4:Transfer injected egg the genome,and about one three into the uterus of a foster mother. ○© eggs that are implanted into a STEP 5:Foster mother gives About 10-30%of off foster mother mouse will have the birth to pups,about 1 in three ain fo h haewye2e38ated es of all their tissues and germ line Bs gene integrated,and will go on Breed mice to produce a baby mouse.Animals STEP 6:Breed transgenic DNA in germ line ropagate that score positive for the human offspring to get homozygous carriers of the transgene. transgene are mated to generate Figure by MIT OCW mice homozygous for the transgene.Among these progeny one is likely to contain the mutated human B-globin protein in its RBCs. This was indeed achieved,BUT,this mouse did not prove to be a good model for sickle cell disease.It turns out that the human B-globin protein does Genotypes of the BsH Transgenic Mice not complex well with the mouse a- globin protein (aM)and so the cloned gene encoding the human a- BM QM M globin protein (aM)was introduced BM QM into fertilized mouse eggs to create a Breed transgenic offspring new transgenic mouse line,which was then mated with the BsH transgenic M QM mouse to produce a mouse expressing both BsH and aM human BM QM proteins. Unfortunately the RBCs of these mice do not Note that the aM gene is almost certain sickle efficiently.....maybe because human a- to integrate into different location than globin is not present. 婴 the BsM gene did,and probably in a BM aM GM different chromosome.These alleles will M aM。aM therefore sort independently when the two transgenic mouse lines are bred Add in the human a-globin transgene and breed mice together.The strong expectation was that the presence of the a"a"BsBs H hemoglobin tetramer in mouse RBCs would lead to the precipitation of fibers
microscope. The male pronucleus is injected (still under the microscope) with purified DNA fragments that contain the βS H gene along with an appropriate promoter region to give it a good chance of being expressed in once integrated into the genome. The injected DNA quite often gets incorporated into the genome, and about one three eggs that are implanted into a foster mother mouse will have the βS H gene integrated, and will go on to produce a baby mouse. Animals that score positive for the human transgene are mated to generate mice homozygous for the transgene. Among these progeny one is likely to contain the mutated human β−globin protein in its RBCs. This was indeed achieved, BUT, this mouse did not prove to be a good model for sickle cell disease. It turns out that the human β-globin protein does not complex well with the mouse α- globin protein (αM) and so the cloned gene encoding the human α- globin protein (αH) was introduced into fertilized mouse eggs to create a new transgenic mouse line, which was then mated with the βS H transgenic mouse to produce a mouse expressing both βS H and αH human proteins. Genotypes of the βS H Transgenic Mice βM βM αM αM αM αM βM βM αM αM αM αM βH S βH S βH S Genotypes of the βS H Transgenic Mice βM βM αM αM αM αM βM βM αM αM αM αM βH S βH S βH S Note that the αH gene is almost certain to integrate into different location than the βS H gene did, and probably in a different chromosome. These alleles will therefore sort independently when the two transgenic mouse lines are bred together. The strong expectation was that the presence of the αH αH βS H βS H hemoglobin tetramer in mouse RBCs would lead to the precipitation of fibers Breed transgenic offspring Add in the human α-globin transgene and breed mice Add in the human α-globin transgene and breed mice Unfortunately the RBCs of these mice do not sickle efficiently……maybe because human α− globin is not present. βM βM αM αM αM αM βH S βH S βM βM αM αM αM αM βH S βH S αH αH Unfortunately the RBCs of these mice do not sickle efficiently……maybe because human α− globin is not present. βM βM αM αM αM αM βH S βH S βM βM αM αM αM αM βH S βH S αH αH βM βM αM αM αM αM βH S βH S αH αH STEP 1: Retrieve fertilized egg from recently mated female mouse. STEP 3: Human “transgene” integrates into the mouse genome at a random site. STEP 4: Transfer injected egg into the uterus of a foster mother. STEP 5: Foster mother gives birth to pups, about 1 in three have the transgene integrated into every cell of its body. STEP 6: Breed transgenic offspring to get homozygous carriers of the transgene. STEP 2: Inject cloned human βS H gene (into male pronucleus). Transfer injected eggs into foster mother Inject foreign DNA into one of the pronuclei Fertilized mouse egg prior to fusion of male and female pronuclei Pronuclei About 10-30% of offspring will contain foreign DNA in chromosomes of all their tissues and germ line Breed mice expressing foreign DNA to propagate DNA in germ line Figure by MIT OCW

and the sickling of the mouse RBCs.However,much to the disappointment of the research teams involved,this was simply not the case.It turns out that the presence of the normal mouse hemoglobin proteins is enough to prevent the mutant hemoglobin tetramers from precipitating into fibers,and so these mice do not make a good model for human sickle cell disease. BM aM GM It was decided that the only solution to this problem would be to eliminate the BM aMaM endogenous mouse a and B globin genes. PROBLEM:These mice still do not have RBCs that sickle very well. The mouse still has mouse a and B globin molecules and their presence is enough to prevent the human hemoglobins from forming Enhamd6ror6deRe8Che2iforhe This will be the topic of the next lecture. SOLUTION:Need to get rid of the endogenous mouse a and B globin genes by targeted homologous recombination to generate "Knock-out"mice
and the sickling of the mouse RBCs. However, much to the disappointment of the research teams involved, this was simply not the case. It turns out that the presence of the normal mouse hemoglobin proteins is enough to prevent the mutant hemoglobin tetramers from precipitating into fibers, and so these mice do not make a good model for human sickle cell disease. PROBLEM: These mice still do not have RBCs that sickle very well. The mouse still has mouse α and β globin molecules and their presence is enough to prevent the human hemoglobins from forming fibers, in much the same way that humans heterozygous for the sickle mutation do not normally have RBCs that sickle. SOLUTION: Need to get rid of the endogenous mouse α and β globin genes by targeted homologous recombination to generate “Knock-out” mice βM βM αM αM αM αM βH S βH S αH αH PROBLEM: These mice still do not have RBCs that sickle very well. The mouse still has mouse α and β globin molecules and their presence is enough to prevent the human hemoglobins from forming fibers, in much the same way that humans heterozygous for the sickle mutation do not normally have RBCs that sickle. SOLUTION: Need to get rid of the endogenous mouse α and β globin genes by targeted homologous recombination to generate “Knock-out” mice βM βM αM αM αM αM βH S βH S αH αH βM βM αM αM αM αM βH S βH S αH αH It was decided that the only solution to this problem would be to eliminate the endogenous mouse α and β globin genes. This will be the topic of the next lecture