
Chap.6 Fe-C Alloy
Chap.6 Fe-C Alloy
outline √Polymorphism of iron Structure and property of each component in Fe-Fe3C diagram. Establishment and anysis of Fe-FeC. The effect of carbon concentration on material structure and property. Application of Fe-Fe3C diagram
Polymorphism of iron Structure and property of each component in Fe-Fe3C diagram. Establishment and anysis of Fe - Fe3C . The effect of carbon concentration on material structure and property. Application of Fe - Fe3C diagram. outline

Carbon(C),Manganese(Mn),Silicon(Si), Phosphorus(P),Sulfur(S) Plain-carbon steel
Carbon (C), Manganese(Mn), Silicon(Si), Phosphorus(P), Sulfur(S) Plain-carbon steel
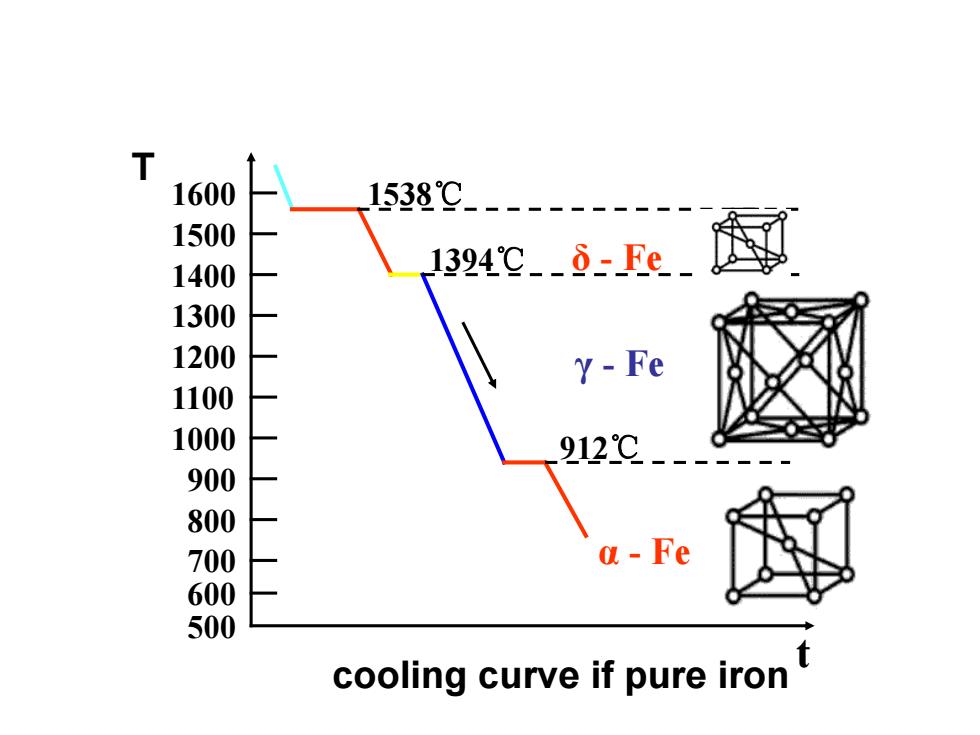
T 1600 1538℃-------- 1500 1400 1324℃-⑧-Fe_ 1300 1200 Y-Fe 1100 出 912℃ a-Fe cooling curve if pure iron
cooling curve if pure iron 1394℃ 1538℃ 1000 600 800 1200 t 1600 1500 500 700 900 1100 1300 1400 912℃ δ - Fe α - Fe γ - Fe T
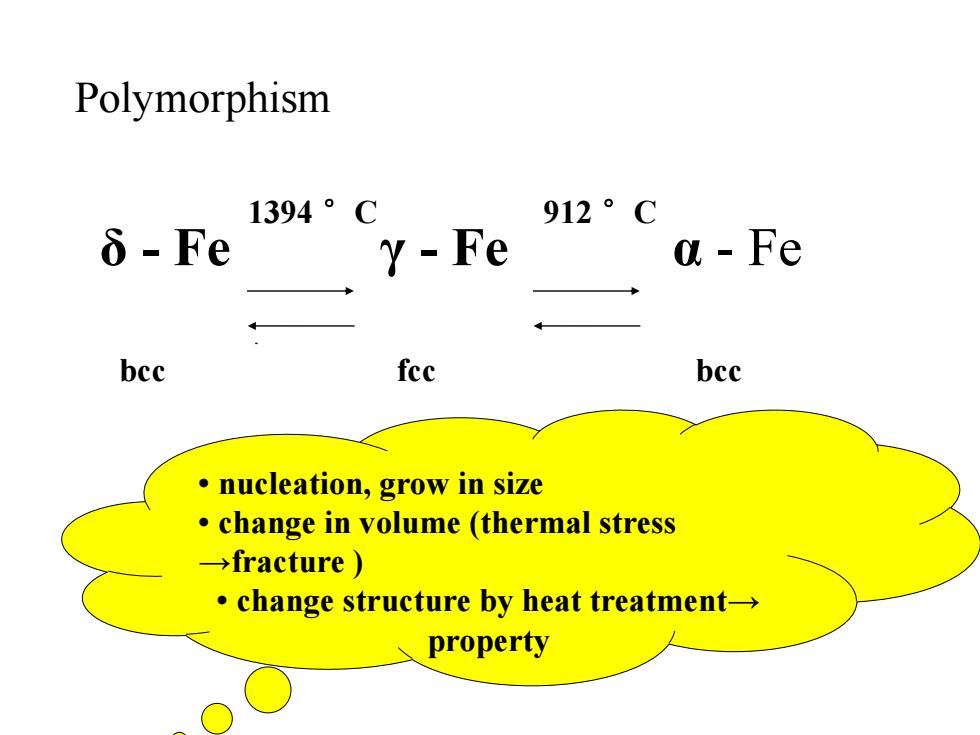
Polymorphism 1394。C 912。C δ-Fe Y-Fe a Fe bcc fec bcc nucleation,grow in size change in volume (thermal stress →fracture) ·change structure by heat treatment-→ property
1394 °C 912 °C bcc fcc bcc δ - Fe γ - Fe α - Fe Polymorphism • nucleation, grow in size • change in volume (thermal stress →fracture ) • change structure by heat treatment→ property
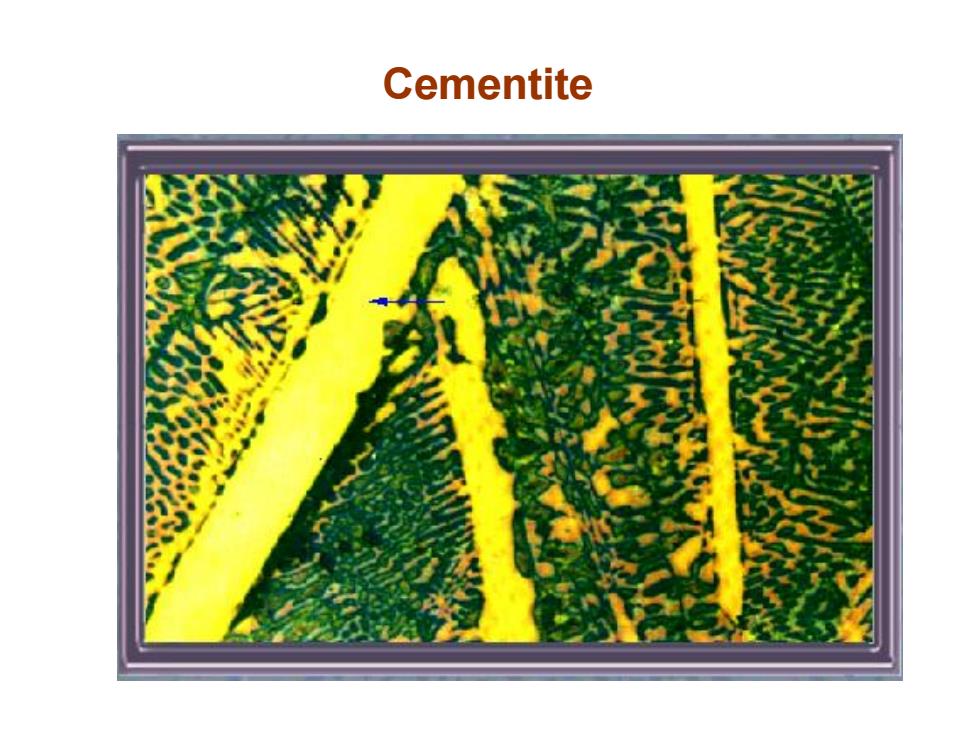
6.1 components and phases in Fe-C alloy The Fe-Fe3C diagram contains the following solid phases:a ferrite,austenite (y),cementite (Fe3C) andδferrite. a ferrite(F):an interstitial solid solution of carbon in a-Fe.Carbon is slightly soluble in a ferrite,the maximum solid solubility is 0.0218%at 727C Austenite(y/A):an interstitial solid solution of carbon in y-Fe.Austenite has a much higher solid solubility for carbon than a ferrite,the maximum solid solubility is 2.11%at 1148C Cementite (Fe3C):the intermetallic compound, with a composition of 6.69%C and 93.1%Fe. Hard and brittle
The Fe-Fe3C diagram contains the following solid phases: α ferrite, austenite (γ), cementite (Fe3C) and δ ferrite. α ferrite(F): an interstitial solid solution of carbon in α-Fe. Carbon is slightly soluble in α ferrite, the maximum solid solubility is 0.0218% at 727℃ Austenite(γ/A): an interstitial solid solution of carbon in γ –Fe. Austenite has a much higher solid solubility for carbon than α ferrite, the maximum solid solubility is 2.11% at 1148℃ Cementite (Fe3C): the intermetallic compound, with a composition of 6.69% C and 93.1% Fe. Hard and brittle. 6.1 components and phases in Fe-C alloy

Austenite
Austenite
Cementite
Cementite
6.2 Fe-Fe3C phase diagram 6.2.1 analysis of Fe-Fe3C phase diagram T Fe Fe3C
Fe T° Fe3C 6.2 Fe - Fe3C phase diagram 6.2.1 analysis of Fe - Fe3C phase diagram
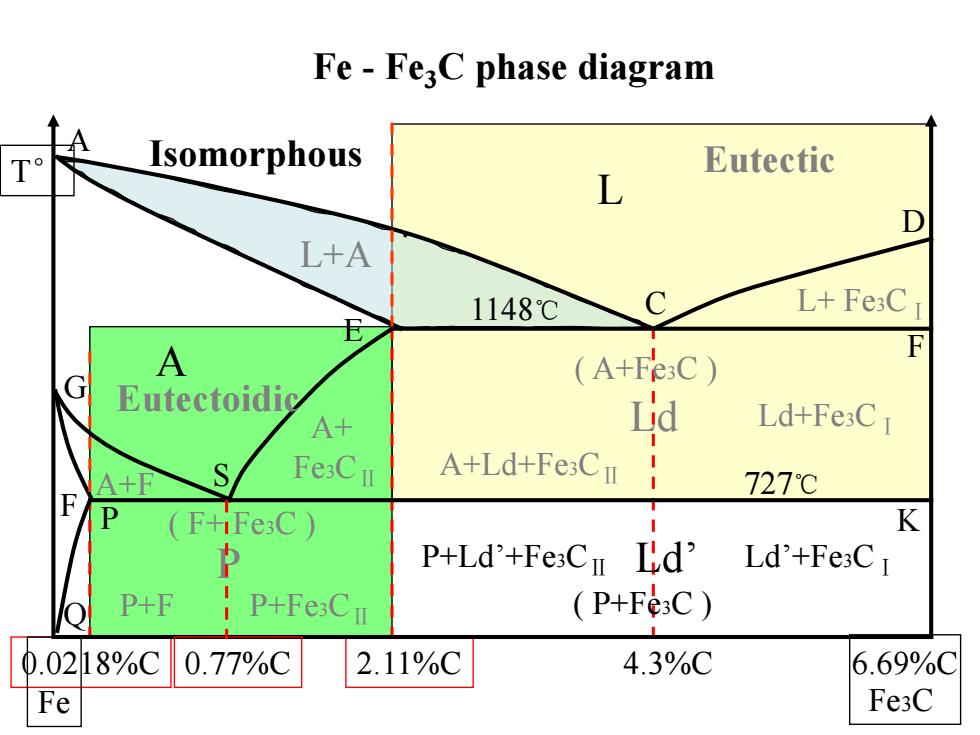
Fe-Fe;C phase diagram T Isomorphous Eutectic L D L+A 1148℃ C L+Fe3C A (A+F3C) F G Eutectoidic A+ a Ld+Fe3CI A+F Fe:CI A+Ld+Fe3CI 727℃ F P (F+iFe3C K 2 P+Ld'+FesCn Ld' Ld'+Fe3CI P+F P+Fe:Cu (P+Fe3C) 0.0218%C 0.77%C 2.11%C 4.3%C 6.69%C Fe Fe3C
Fe - Fe3C phase diagram A C D E F G S P Q 1148℃ 727℃ L A L+A L+ Fe3CⅠ 0.0218%C 2.11%C 4.3%C 6.69%C Fe Fe3C T° ( A+Fe3C ) Ld Ld+Fe3CⅠ A+Ld+Fe3CⅡ F A+F A+ Fe3CⅡ ( F+ Fe3C ) P P+F 0.77%C P+Fe3CⅡ P+Ld’+Fe3CⅡ Ld’ Ld’+Fe3CⅠ K Eutectic Eutectoidic Isomorphous ( P+Fe3C )