
Introduction to Materials Module 2 Nature Structure of Materials PDF文件使用"pdfFactory Pro”试用版本创建www,fineprint.com.cn
Introduction to Materials Module 2 Nature & Structure of Materials PDF 文件使用 "pdfFactory Pro" 试用版本创建 www.fineprint.com.cn

2.2 Crystalline structure PDF文件使用"pdfFactory Pro”试用版本创建ww,fineprint..com,cn
2.2 Crystalline structure a b c x y z a b g PDF 文件使用 "pdfFactory Pro" 试用版本创建 www.fineprint.com.cn
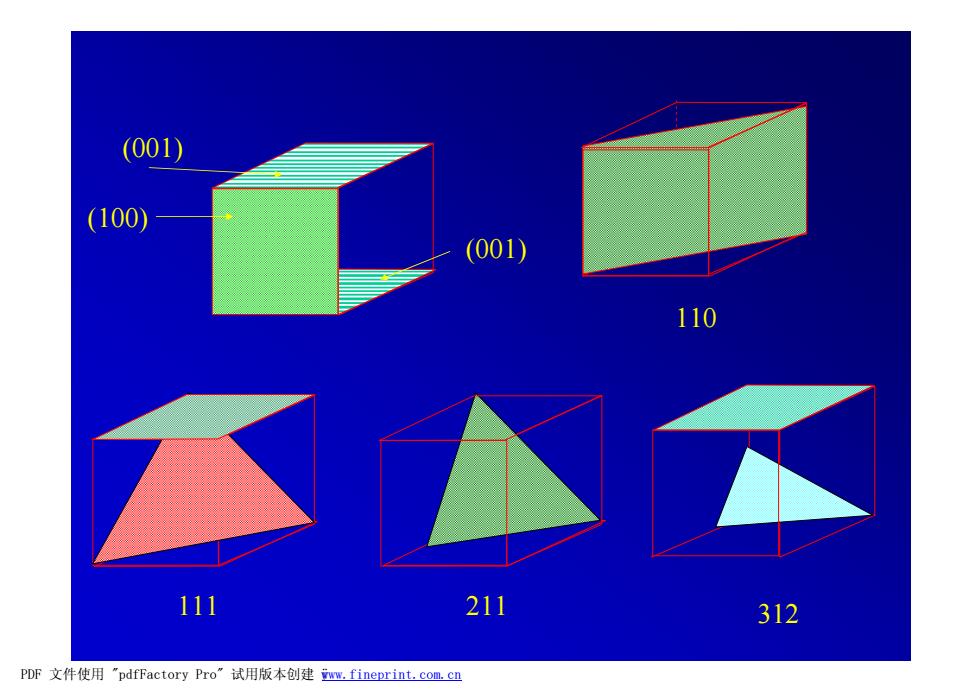
(001) (100) (001) 110 111 211 312 PDF文件使用"pdfFactory Pro”试用版本创建ww,fineprint.com.cn
(100) (001) (001) 111 211 110 312 PDF 文件使用 "pdfFactory Pro" 试用版本创建 ÿwww.fineprint.com.cn
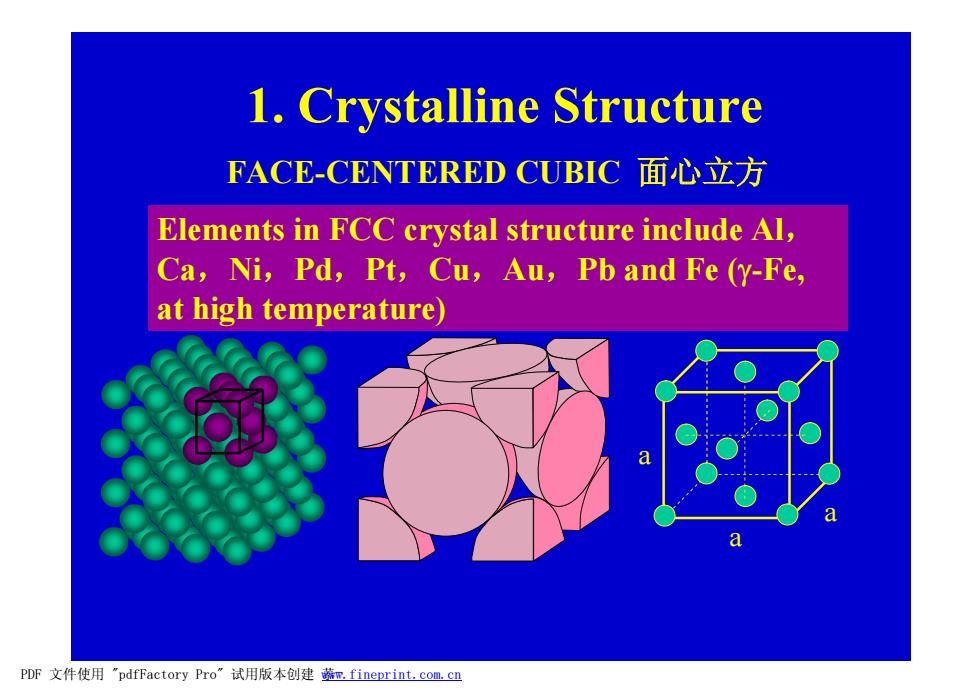
1.Crystalline Structure FACE-CENTERED CUBIC面心立方 Elements in FCC crystal structure include Al, Ca,Ni,Pd,Pt,Cu,Au,Pb and Fe (y-Fe, at high temperature) a a PDF文件使用"pdfFactory Pro”试用版本创建蔬m.fineprint.com.cn
1. Crystalline Structure FACE-CENTERED CUBIC 面心立方 a a a Elements in FCC crystal structure include Al, Ca,Ni,Pd,Pt,Cu,Au,Pb and Fe (g-Fe, at high temperature) PDF 文件使用 "pdfFactory Pro" 试用版本创建 菾¬ www.fineprint.com.cn
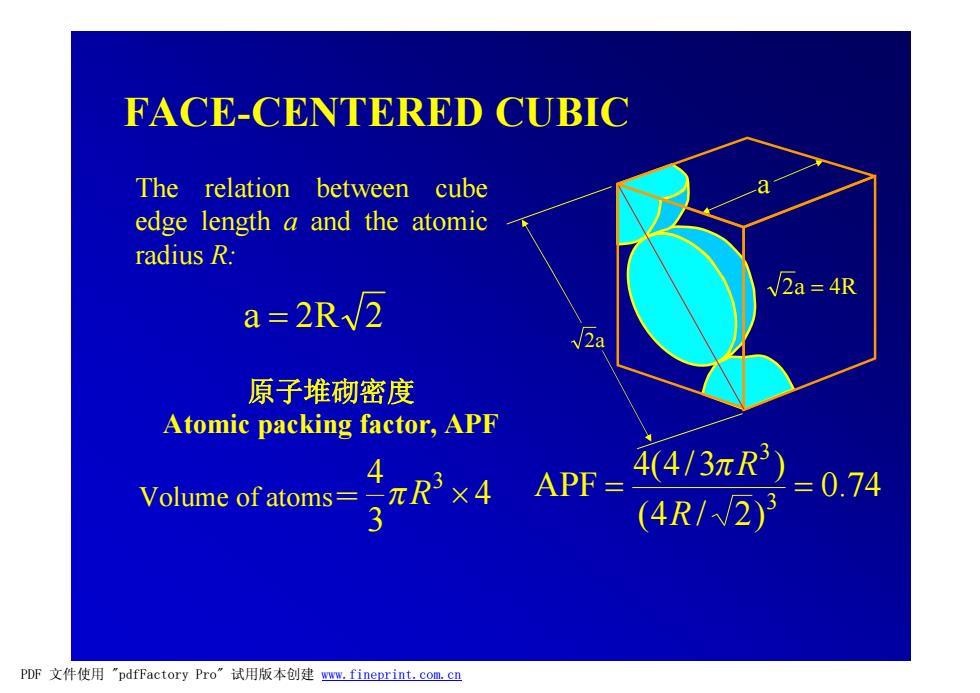
FACE-CENTERED CUBIC The relation between cube edge length a and the atomic radius R. v2a 4R a=2R√2 2a 原子堆砌密度 Atomic packing factor,APF Volume of atoms= x4 4 APF= 4413元R)=0.74 (4R/√2)3 PDF文件使用"pdfFactory Pro”试用版本创建www,fineprint.com.cn
FACE-CENTERED CUBIC 原子堆砌密度 Atomic packing factor, APF 0.74 (4 / 2) 4(4/3 ) APF 3 3 = = R pR 4 3 4 3 Volume of atoms= pR ´ a 2a 2a = 4R a = 2R 2 The relation between cube edge length a and the atomic radius R: PDF 文件使用 "pdfFactory Pro" 试用版本创建 www.fineprint.com.cn

BODY-CENTERED CUBIC 体心立方 Under conventional conditions,elements in BCC crystal structure include Li,Na,K,and transition metals such as V,Cr,Nb,Mo,Ta,W and Fe (a-Fe),etc. a PDF文件使用"pdfFactory Pro”试用版本创建E.fineprint..com,cn
BODY-CENTERED CUBIC 体心立方 a a a Under conventional conditions, elements in BCC crystal structure include Li,Na,K, and transition metals such as V,Cr,Nb,Mo,Ta,W and Fe (a-Fe), etc. PDF 文件使用 "pdfFactory Pro" 试用版本创建 ÿÿwww.fineprint.com.cn f
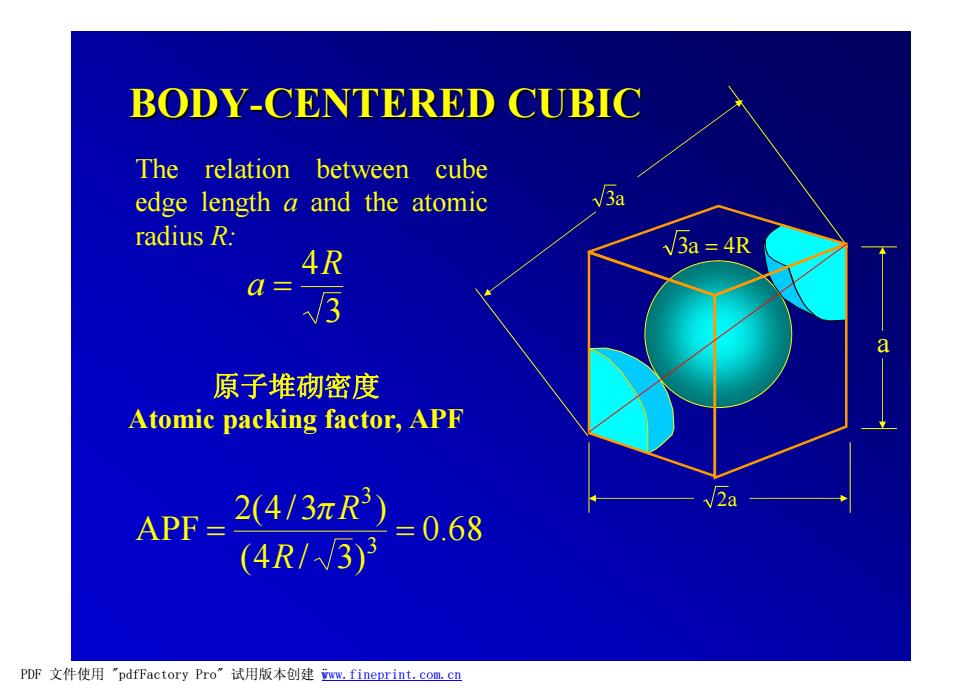
BODY-CENTERED CUBIC The relation between cube edge length a and the atomic V3a radius R. 4R 3a =4R a=3 Q 原子堆砌密度 Atomic packing factor,APF APF= (4/3πR3) 2a =0.68 (4R/3)3 PDF文件使用"pdfFactory Pro”试用版本创建ww,fineprint.com.cn
3a 3a = 4R 2a a BODY-CENTERED CUBIC 原子堆砌密度 Atomic packing factor, APF 3 4R a = 0.68 (4 / 3) 2(4/3 ) APF 3 3 = = R pR The relation between cube edge length a and the atomic radius R: PDF 文件使用 "pdfFactory Pro" 试用版本创建 ÿwww.fineprint.com.cn
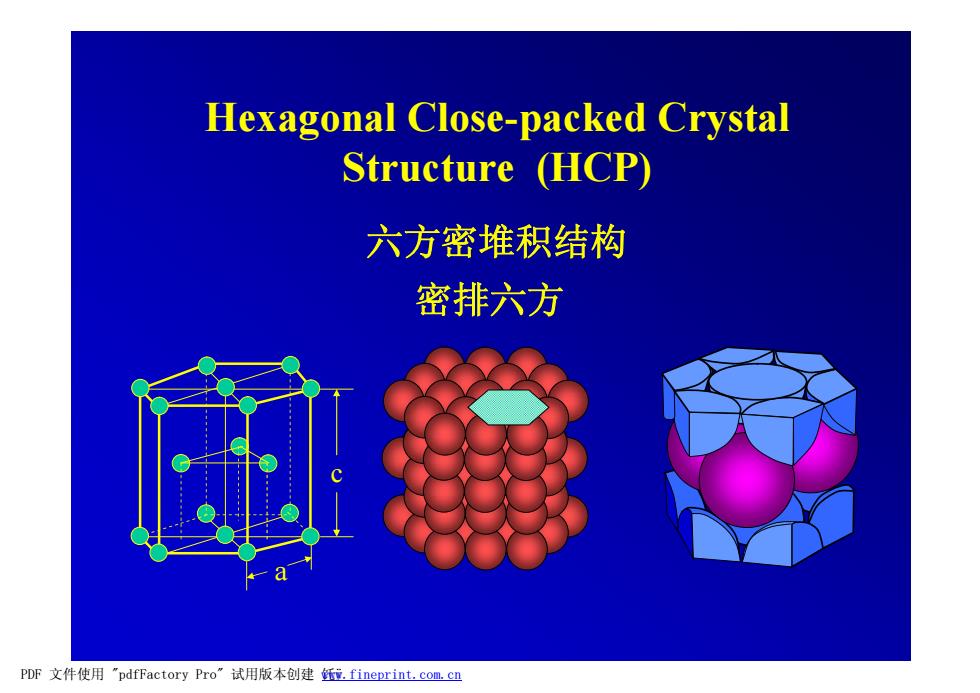
Hexagonal Close-packed Crystal Structure (HCP) 六方密堆积结构 密排六方 PDF文件使用"pdfFactory Pro”试用版本创建f.fineprint.com.cn
Hexagonal Close-packed Crystal Structure (HCP) a c 密排六方 六方密堆积结构 PDF 文件使用 "pdfFactory Pro" 试用版本创建 饦ÿ www.fineprint.com.cn
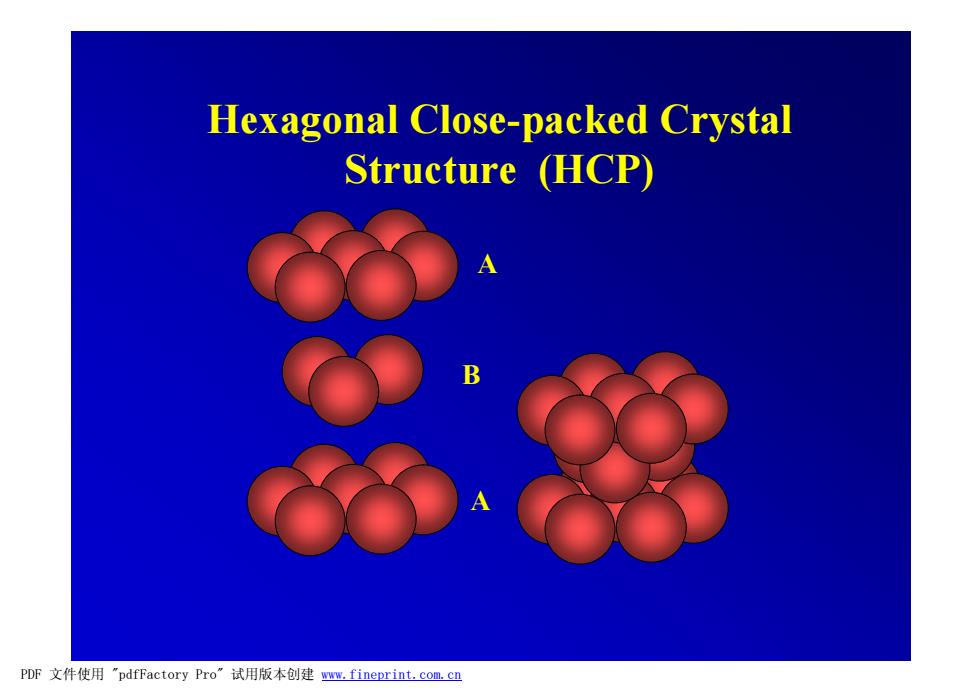
Hexagonal Close-packed Crystal Structure (HCP) PDF文件使用"pdfFactory Pro'”试用版本创建wm,fineprint.com,cn
A B A Hexagonal Close-packed Crystal Structure (HCP) PDF 文件使用 "pdfFactory Pro" 试用版本创建 www.fineprint.com.cn

PDF文件使用"pdfFactory Pro”试用版本创建ww,fineprint.com.c迎
PDF 文件使用 "pdfFactory Pro" 试用版本创建 www.fineprint.com.cn